Sino Biological is an international reagent supplier and service provider, specializing in recombinant protein production and antibody development. Our manufacturing site is ISO 9001:2015 and ISO13485:2016 certified, which indicates our commitment to ensuring our products meet quality requirements and customer expectations.
Our scientists have developed 4,000+ IHC validated antibodies suitable for immunohistochemistry application, from pathology and cancer research to immunology and usual biomarkers. All of these antibodies are fully validated in various human or animal tissue slides.
ISO9001:2005 and ISO13485:2016 Certified
What makes a good antibody for immunohistochemistry? Finding the best antibody that works for specific target can be very challenging. This is partly because not all suppliers fully validate their antibodies.
As a trusted antibody manufacturer, Sino Biological is dedicated to providing you with only the highest quality antibodies. All our antibodies are rigorously validated and are characterized in several applications. After a series of stringent screening processes, our IHC antibodies exhibit excellent performances in antigen recognition, localization, specificity, affinity, etc. This can ensure you get high-quality images for publication.
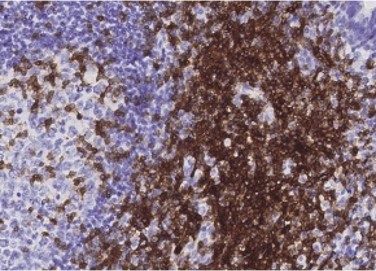
Tonsil
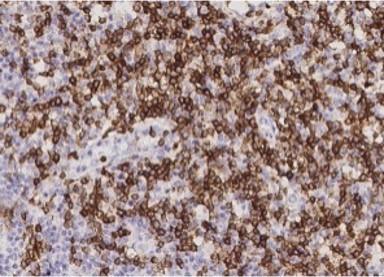
Submaxillitis
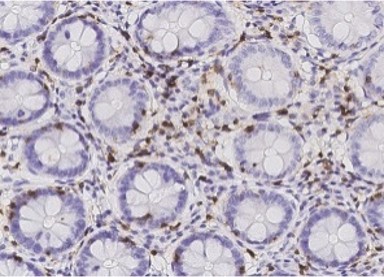
Colon
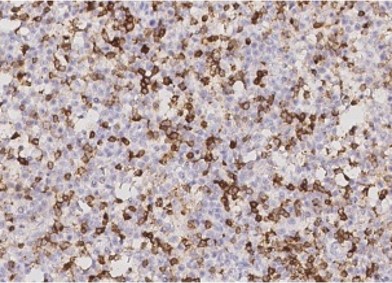
Lymphoma
Lung
Testis
Skeletal Muscle
Brain
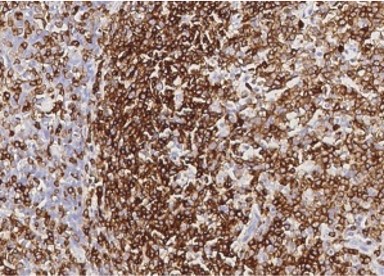
Tonsil
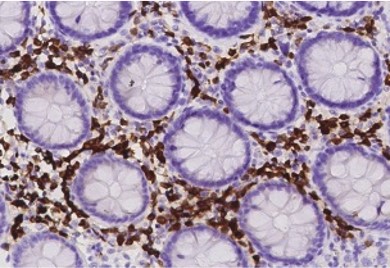
Colon
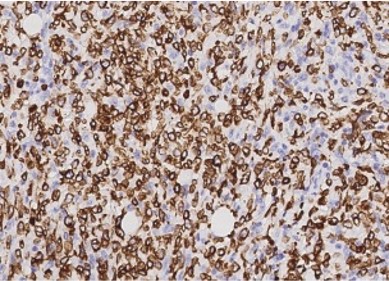
Lymphoma
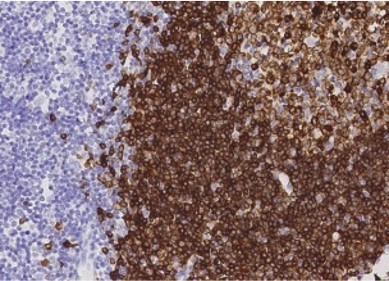
Lymph Node
Brain
Testis
Skeletal Muscle
Thyroid
As a leading supplier in antibody development, Sino Biological is committed to producing the highest quality IVD raw materials and reagents. Our antibodies have been tested and validated for immunohistochemistry applications on formalin-fixed paraffin embedded (FFPE) normal or pathological tissues from multiple donors.
Each IHC antibody is manufactured in-house, and undergoes rigorous quality control testing to ensure reproducible and reliable immunohistochemistry test results.
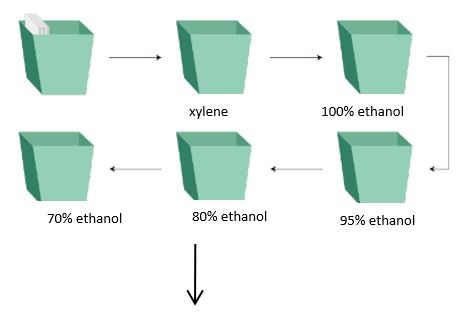
Wash slides with specific reagents in the following order:
Excellent IHC staining is a foundation of correct judgment for staining, since there are many steps and links during the whole immunohistochemistry process, each of which is likely to affect the final result of the staining, and it is not very easy to do an immunohistochemistry quality slice.
There are often incorrect signals except for those correct positive signals in immunohistochemical staining. Such incorrect staining is called non-specific staining. There are a variety of causes resulting in non-specific staining, which are summarized as follows:
1. How to Choose Antibody for IHC Experiments, the Monoclonal or Polyclonal Antibodies?
The characteristics of monoclonal and polyclonal antibodies determine their strengths and weaknesses. Monoclonal antibodies are a homogeneous population of immunoglobulin directed against a single epitope. They are generated by a single B-cell clone from one animal and consequently are immunochemically similar. Polyclonal antibodies are a heterogeneous mixture of antibodies directed against various epitopes of the same antigen. They are generated by different B-cell clones of one animal and consequently are immunochemically dissimilar. The antibodies in a polyclonal mixture can have slightly different specificities and affinities.
If the protein target has the same amino acid sequence, a monoclonal antibody is more appropriate. Once antigen conformational changes are produced due to various reasons, such as the interaction of proteins, post-translational modification, temperature, pH value, fixed, and salt concentration, the combined effect of monoclonal antibody and antigen will be seriously affected. Because polyclonal antibodies have multiple epitopes, they are not affected by changes in protein conformation, in general, within a certain range of pH and salt concentration, and thus the antigen-binding with a polyclonal antibody is more stable than with a monoclonal antibody.
2. How to Choose Incubation Time for Primary Antibody in IHC?
The concentration of the primary antibody, incubation time, and temperature will affect the quality of the immunohistochemical staining. These variables need to be optimized for different antibodies, in order to achieve high specificity and low background immunohistochemical staining. The optimization process is generally to maintain a fixed incubation time and temperature, while varying the concentration of the antibody. For example, high-affinity antibodies may be used with a relatively high concentration of antibody with shortened incubation time, or a relatively low concentration antibody with longer incubation time. In order to obtain highly specific staining, a longer incubation time and lower incubation temperatures are often chosen.
3. Antigen Retrieval-Should I do PIER or HIER?
Antigens can be masked as a result of the fixation process, which makes antibody binding impossible. The masking can be reversed with a technique called antigen retrieval/antigen unmasking, which is mediated either by heat (HIER; heat-induced antigen retrieval) or with proteases (PIER; proteolytic-induced antigen retrieval). The HIER method acts by restoring the secondary and tertiary structure of epitopes, while the PIER method acts by degrading the peptides which mask the epitopes with enzymes, such as proteinase K, trypsin, and pepsin. However, PIER may also result in alterations to the specimen morphology or the antigen itself. Consequently, PIER is less frequently used than HIER.
To determine whether antigen retrieval should be performed and by which method, the following guidelines can be followed.