Latest News
Introduction to R programming for life-scientists
Free online workshop: 7th July2022
R is widely used for analysis of biological data sets. However, R is complex and requires experience to use effectively. Using a real data set from his research, Dr Toryn Poolman from UCL will take us through some basic functions of R that will be useful to all life-scientists.
The workshop will include:
- Statistical analysis of a real-life data set
- Creating a multi-panel figure
- Adding in stats to the figure
- Annotating graphs using R markdown

Dr Toryn Poolman (@toryn13)
University College London
Abbexa achieves ISO 9001 Accreditation
Abbexa has successfully achieved its accreditation to ISO 9001:2015 quality standard in recognition of the company’s ongoing focus on quality.

Best practice recommendation from the auditors

This accreditation establishes Abbexa’s commitment to continually improve its products, services and processes. Abbexa has developed clearly defined procedures in all business areas and strives to maintain a high level of quality and strong customer service, and ongoing investment in development.
Antibody Engineering and Therapeutics Europe
We're excited to be taking part at this year's Antibody Engineering & Therapeutics Europe. Have you reserved your seat?
Antibody Engineering & Therapeutics Europe, returns in a hybrid format7-9 June, 2022(in Amsterdam + 100% digitally live-streamed). We would encourage you to downloadthe full agenda and take a look at the prestigious line-up of speakers that will be taking part in this year's programme, if you haven't done so already.
Keynote speakers include:
- Ulrike Philippar, Ph.D.,Senior Director Oncology, Global Head of Discovery Hematological Malignancies,Janssen R&D
- Jane Osbourn, OBE FMed Sci,Chief Scientific Officer,Alchemab Therapeutics
- Pierre Bruhns, Ph.D.,Director, Unit of Antibodies in Therapy & Pathology,Institut Pasteur
- Chris Martin, DPhil,CEO, Co-Founder, and Director,ADC Therapeutics
Immuno-Oncology : The Latest Frontier in Cancer Therapy

For as long as medicine has existed, cancers have ravaged humanity. The search for an eventual cure has been a tremendous & tireless pursuit, selflessly undertaken by doctors, researchers, scientists & the entire healthcare community. With each passing year and the availability of sophisticated technologies, their efforts continue to go from strength to strength, expanding our means to adequately cope, manage and possibly rebuff the disease which has so often reminded us of our mortality. Up until recently, Chemotherapy, Surgeries, Radiation & Targeted Drug therapies had been the go-to for… read more
CRISPR-Cas Systems Gain Local Flexibility, Retain Global Specificity
In genome editing, it is possible to have too much of a good thing—a good thing known as specificity. Usually, specificity is much desired. It helps minimize the potential for off-target effects. However, specificity can have a downside. It can make it harder to recognize DNA sequences that contain naturally occurring polymorphisms.
Consider the practical implications. For example, if a genome editing system is highly specific, it may target a particular gene while ignoring slightly different variations of that gene. Also, in diagnostic applications, genome editing tools may be overly precise, detecting just one of several closely related pathogens.
Major CRISPR patent decision won’t end tangled dispute
A long-running dispute between two groups that claim to have invented the revolutionary CRISPR–Cas9 gene-editing tool is likely to remain unresolved for years to come, lawyers say — despite the US patent office’s latest decision to award key patent rights to one of the teams.
The ultimate outcome of the patent row — which began in 2016 — could mean millions of dollars in royalties for the victor, if and when CRISPR-based therapies make it to market.
Simple DNA test could detect common neurological disorders, study says
Simple DNA test could detect common neurological disorders, study says
Exclusive: whole genome sequencing could end ‘diagnostic odyssey’ of multiple tests that still do not give precise diagnosis

The new test utilises an algorithm that can spot repetitive elements in whole genome sequences, by comparing those from healthy people with those affected by repeat expansion disorders.Photograph: nobeastsofierce Science/Alamy
A simple test could end years of uncertainty for people with relatively common neurological conditions, new research has found.
Historically, obtaining a definitive diagnosis for conditions including Huntingdon’s disease and some forms of amyotrophic lateral sclerosis has been difficult, because, although the cause of the symptoms is genetic, knowing which test to carry out has resulted in delays of many years.
Now, a new study suggests that whole genome sequencing (WGS) can quickly and accurately detect the most common inherited neurological disorders, and could be implemented in routine clinical practice with immediate effect.
Click here to read more
Breakthrough Using CRISPR to Target Fat Cells in Genetic Study of Obesity
Breakthrough Using CRISPR to Target Fat Cells in Genetic Study of Obesity
Fat—it is vital for life but too much can lead to a host of health problems. Studying how fat tissue, or adipose, functions in the body is critical for understanding obesity and other issues.
But structural differences in fat cells and their distribution throughout the body make doing so challenging.
“Fat cells are different from other cells in that they lack unique cell surface receptors and only account for a minority of the cells within fat tissue,” said Steven Romanelli, Ph.D., from the Department of Molecular & Integrative Physiology at the University of Michigan.
In a new paper published in the Journal of Biological Chemistry, Romanelli, Ormand MacDougald, Ph.D. and their colleagues describe a breakthrough using CRISPR-Cas9, a tool that has transformed molecular biological research, but whose use in the study of adipose tissue had been elusive.
Deep learning dreams up new protein structures
Deep learning dreams up new protein structures
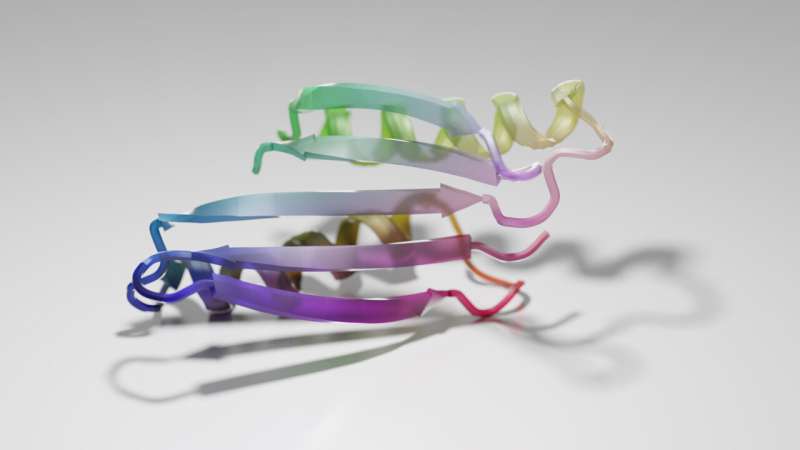
This twisting protein structure is one of hundreds dreamed up by a machine-learning algorithm. Credit: Ian C. Haydon/UW Medicine Institute for Protein Design
Just as convincing images of cats can be created using artificial intelligence, new proteins can now be made using similar tools. In a report inNature, researchers describe the development of a neural network that "hallucinates" proteins with new, stable structures.
Proteins, which are string-like molecules found in every cell, spontaneously fold into intricate three-dimensional shapes. These folded shapes are key to nearly every biological process, including cellular development, DNA repair, and metabolism. But the complexity of protein shapes makes them difficult to study. Biochemists often use computers to predict how protein strings, or sequences, might fold. In recent years, deep learning has revolutionized the accuracy of this work.
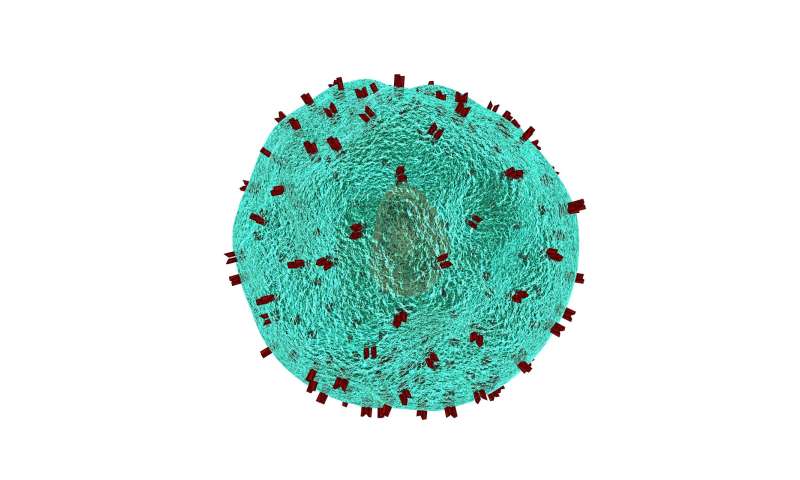
SARS-CoV-2 uses sugars to invade human cells
SARS-CoV-2 uses sugars to invade human cells
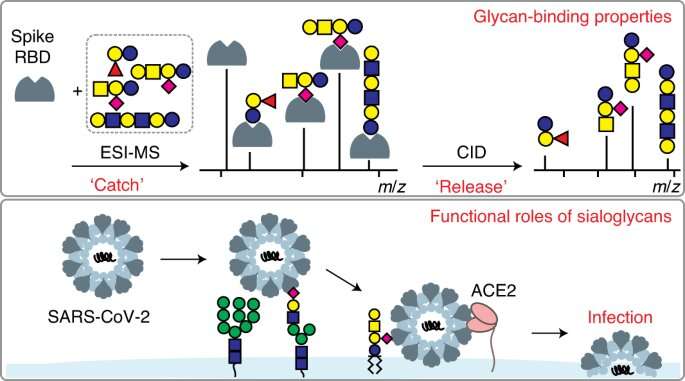
Graphical abstract. Credit: DOI: 10.1038/s41589-021-00924-1
Sugars found on the surface of human cells influence COVID-19 infection, according to a University of Alberta-led study that is one of the first to observe this relationship and suggests that cells in the brain might be particularly susceptible.
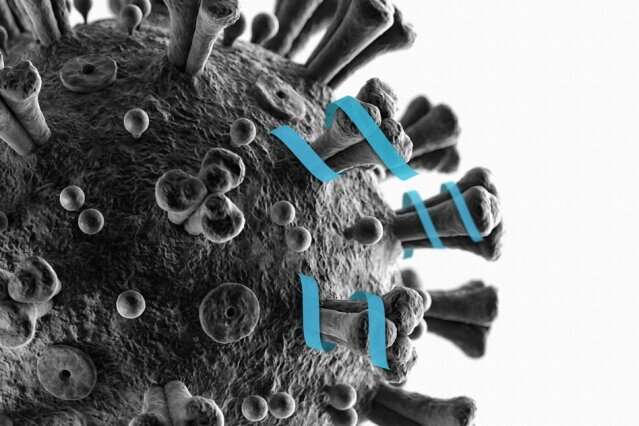
Scientists discover a highly potent antibody against SARS-CoV-2
Scientists discover a highly potent antibody against SARS-CoV-2
Scientists at Lausanne University Hospital (CHUV) and EPFL have discovered a highly potent monoclonal antibody that targets the SARS-CoV-2 spike protein and is effective at neutralizing all variants of concern identified to date, including the delta variant. Their findings are published in the prestigious journalCell Reports.
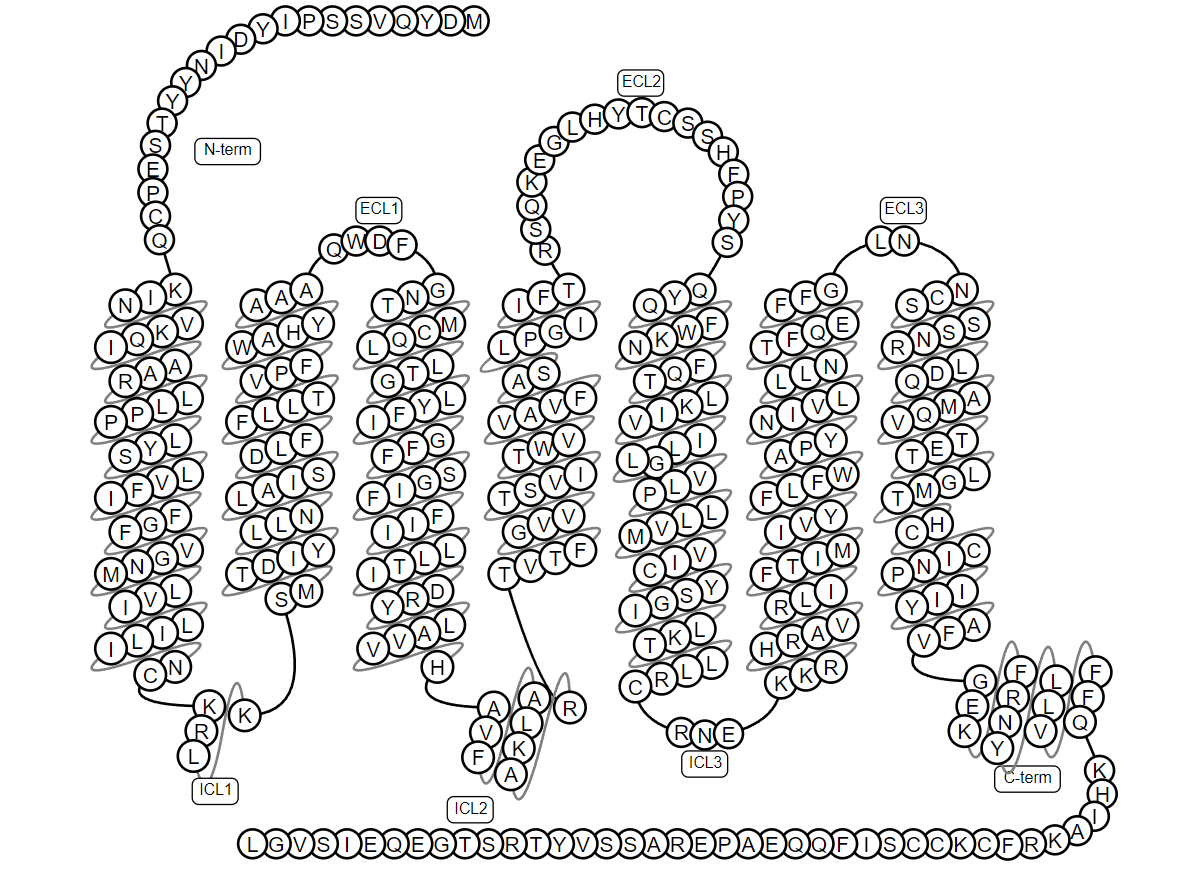
GPCRs: From signal pathways to targeted drug development Acro Biosystems

Many membrane proteins present on the cell membrane, responsible for cell protection, internal and external material transportation, and signal transmission. The diversity in the functions of membrane protein has also made it an important target in the field of drug development. More than 50% of drugs currently approved target human membrane proteins. G protein-coupled receptors (GPCRs) are the largest family of membrane proteins. The primary function of GPCRs is to transduce a wide and diverse array of extracellular stimuli such as biogenic amines, peptides, hormones, neurotransmitters,
King Abdullah University of Science & Technology Joins Kerafast as 200th Providing Institution
King Abdullah University of Science & Technology Joins Kerafastas 200th Providing Institution
Achievement reflects interest of researchers to offer scientific research reagents online
Boston, Mass., August 17, 2021. Kerafast, Inc. announced today that King Abdullah University of Science and Technology (KAUST), located in Thuwal, Saudi Arabia, is the 200thacademic institution to join their international community of scientists. Kerafast is the developer of an online portal for academic researchers worldwide to share their laboratory-created reagents. The company specializes in providing access to difficult-to-obtain materials, while providing generous royalties to the investigators and their institutions. The KAUST addition brings new biomaterials to the catalog, including nanopeptide reagents used for biomedical applications.
Read More click here
CRISPR therapies march into clinic, but genotoxicity concerns linger
CRISPR therapies march into clinic, but genotoxicity concerns linger
Following reports of collateral damage caused by CRISPR genome editing, now chromothripsis, a phenomenon associated with cancer, enters the spotlight.

Chromosomes in dividing cells can undergo chromothripsis, which literally means ‘chromosome shattering’.Credit: Zoonar GmbH / Alamy Stock Photo
A recent study has identified another potential hazard for developers of genome editing therapies based on CRISPR–Cas9. The double-strand DNA breaks introduced during CRISPR editing could result in chromothripsis, an extremely damaging form of genomic rearrangement that results from the shattering of individual chromosomes and the subsequent rejoining of the pieces in a haphazard order. Although most cells do not remain viable after undergoing such a dramatic alteration, those that do could, in theory, express oncogenic fusion proteins or give rise to dysregulated expression of particular genes that could cause problems
‘Super-antibodies’ could curb COVID-19 and help avert future pandemics
‘Super-antibodies’ could curb COVID-19 and help avert future pandemics
Companies are designing next-generation antibodies modeled on those taken from unique individuals whose immune systems can neutralize any COVID-19 variant—and related coronaviruses, too.
The US Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) in late May to sotrovimab, providing a new therapeutic weapon in the fight against SARS-CoV-2—and future coronaviruses with pandemic potential.
According to analysts and researchers alike, so-called super-antibodies such as sotrovimab should have an edge over first-generation monoclonal antibody (mAb) therapies for COVID-19 because of their broad neutralization capacity in the face of emerging virus variants. “Physicians aren’t going to sequence what version of the virus people have, so they’ll go for the antibodies that have the higher barrier to resistance or the ones that work on [known] variants,” says Phil Nadeau, an analyst at Cowen.
CRISPR-Cas components work together to enhance protection from viruses
CRISPR-Cas components work together to enhance protection from viruses
Researchers from Skoltech and their colleagues from Russia and the U.S. have shown that the two components of the bacterial CRISPR-Cas immunity system, one that destroys foreign genetic elements such as viruses and another that creates "memories" of foreign genetic elements by storing fragments of their DNA in a special location of bacterial genome, are physically linked. This link helps bacteria to efficiently update their immune memory when infected by mutant viruses that learned to evade the CRISPR-Cas defense. The paper was published in the journalProceedings of the National Academy of Sciences.
ChromoTek and Absolute Antibody Collaborate on Recombinant Engineered Antibodies
ChromoTek and Absolute Antibody Collaborate on Recombinant Engineered Antibodies
Nanobody-based chimeric antibodies target fluorescent and structural proteins
Munich, Germany and Redcar, UK: February 25, 2021.ChromoTek GmbH part of Proteintech specializing in Nanobody-based reagents, and Absolute Antibody Ltd., a leading provider of recombinant antibody technology, today announced a partnership to offer recombinant engineered antibodies to scientists worldwide. The chimeric antibodies were originally derived from alpaca Nanobodies and engineered onto mouse and rabbit Fc domains to open up new research applications. Available antibodies target key fluorescent proteins commonly used as tags for protein visualization, as well as the structural protein vimentin.

Two variants have merged into heavily mutated coronavirus
Two variants have merged into heavily mutated coronavirus
The UK and California variants of coronavirus appear to have combined into a heavily mutated hybrid, sparking concern that we may be entering a new phase of the covid-19 pandemic
Two variants of the SARS-CoV-2 coronavirus that causes covid-19 have combined their genomes to form a heavily mutated hybrid version of the virus. The “recombination” event was discovered in a virus sample in California, provoking warnings that we may be poised to enter a new phase of the pandemic.
The hybrid virus is the result ofrecombination of the highly transmissible B.1.1.7 variant discovered in the UK and the B.1.429 variant that originated inCalifornia and which may beresponsible for a recent wave ofcases in Los Angeles because itcarries a mutation making it resistant to some antibodies.
SARS-CoV-2 needs cholesterol to invade cells and form mega cells
SARS-CoV-2 needs cholesterol to invade cells and form mega cells
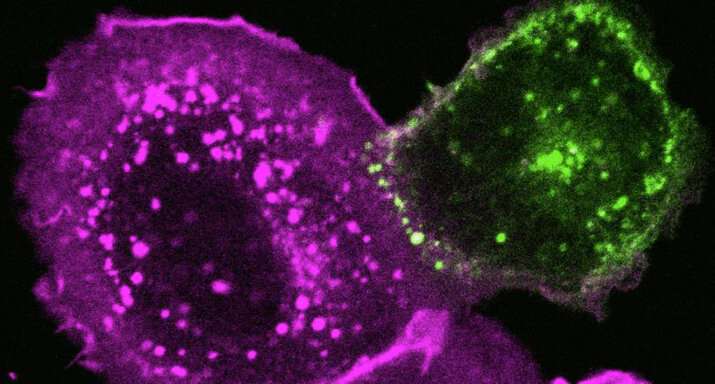
Researchers engineered cells to carry either a protein (green) from SARS-CoV-2 or its human target ACE2 (magenta). When near each other, the cells’ membranes fused. Researchers think a similar process lets the virus slip into cells. Credit: D. Sanders et al./bioRxiv.org
People taking cholesterol-lowering drugs may fare better than others if they catch the novel coronavirus. A new study hints at why: the virus relies on the fatty molecule to get past the cell's protective membrane.
To cause COVID-19, the SARS-CoV-2 virus must force its way into people's cells—and it needs an accomplice. Cholesterol, the waxy compound better known for clogging arteries, helps the virus open cells up and slip inside, Howard Hughes Medical Institute Investigator Clifford Brangwynne's lab reports
Most patients hospitalised with COVID-19 have at least 1 symptom 6 months after falling ill:
Most patients hospitalised with COVID-19 have at least 1 symptom 6 months after falling ill: Wuhan study
More than three quarters of COVID-19 patients have at least one ongoing symptom six months after initially becoming unwell, according to research published inThe Lancet.
The cohort study, looking at long-term effects of COVID-19 infection on people hospitalised in Wuhan, China, reveals that the most common symptom to persist is fatigue or muscle weakness (63% of patients), with patients also frequently experiencing sleep difficulties (26%). Anxiety or depression was reported among 23% of patients.
Click here to read more
LSBIO ACQUIRES ABSOLUTE ANTIBODY LTD
Seattle - (December 22, 2020) – LSBio (LSBio™), a leading provider of antibodies and life science research reagents, is pleased to announce its acquisition of Redcar, UK-based Absolute Antibody, Ltd. and its sister company Kerafast. Terms of the transaction were not disclosed.
LSBio provides IHC-validated reagent antibodies and services to the global community of academic, pharmaceutical and biotech researchers. The Company has built a catalog of more than 750,000 reagent antibodies, assay kits, proteins, and biochemicals, and its acquisition of Absolute Antibody and Kerafast further expands its product portfolio by adding a range of unique reagents and antibody services.
Absolute Antibody specializes in recombinant antibody technology, offering antibody sequencing, engineering and recombinant production as custom services, as well as a unique catalog of recombinant antibodies and Fc Fusion proteins, engineered into new and useful formats. In 2018, Absolute Antibody merged with Kerafast Inc., a Boston-based company that facilitates access to unique laboratory-made reagents, to further the availability of recombinant antibodies. The new acquisition combines a strong recombinant antibody portfolio with LSBio’s expertise in application-specific validation to make sequence-defined, reproducible antibodies more widely available for IHC and other key research applications.
“We are very excited to add Absolute Antibody and Kerafast into the LSBio family of companies,” said Dr. Heather Holemon, CEO of LSBio. “It provides an opportunity to offer more to our customers by adding their recombinant antibody production and engineering capabilities as well as their highly complementary product portfolio. This transaction aligns with our goal of providing researchers access to what they are asking for as far as superior consistency and better validation.”
“We are delighted to be joining forces with the LSBio team,” said Dr. Nicholas Hutchings, founder and CEO of Absolute Antibody. “Absolute Antibody was founded with a vision to make engineered recombinant antibodies accessible to all, and we have grown rapidly as the demand for recombinant antibody technology has increased. That vision remains and working with LSBio enables us to continue growing both the recombinant antibody catalog and our custom services, while still delivering the highest level of product quality and customer satisfaction.”
The acquisition marks the latest investment by LSBio to rapidly build its product portfolio. In 2019, LSBio acquired both Nordic-MUbio and Everest Biotech to strengthen the Company’s offering of proprietary monoclonal and polyclonal antibodies. All LSBio companies currently retain their independent names, websites and employees, with the entire family of companies owned by St. Louis-based private equity firm Thompson Street Capital Partners.
About LSBio
LSBio (www.lsbio.com) enables researchers by providing a variety of antibodies and research reagents. Previous acquisitions of Nordic-MUbio and Everest Biotech have strengthened the company’s offering of proprietary monoclonal and polyclonal antibodies. The continued emphasis for LSBio has been on building the portfolio and coupling that with application-specific antibody validation. Through ongoing in-house testing, over 15,000 of the LSBio antibodies have been identified as exceptional IHC reagents and given the IHC-plus™ brand. LSBio itself was acquired by Thompson Street Capital Partners (TSCP) in 2018.
About Absolute Antibody
Absolute Antibody (www.absoluteantibody.com) was founded in 2012, with the vision to make engineered recombinant antibodies available to all. The company offers antibody sequencing, engineering and recombinant production as custom services, as well as a unique catalog of engineered recombinant antibodies. Absolute Antibody customers include leading pharmaceutical, biotechnology and diagnostics companies, as well as academic researchers in more than 60 different countries worldwide.
About Kerafast
Kerafast, Inc. (www.kerafast.com) is a reagent company whose primary mission is to make unique laboratory-made research tools easily accessible to the global scientific community. Since its founding in 2011, Kerafast has partnered with more than 190 academic research institutions internationally and provided reagents to scientists in 63 countries across six continents. In 2018, Kerafast merged with Absolute Antibody, a company specializing in recombinant antibody technology, to further improve the selection of research tools available to the scientific community.
About TSCP
Thompson Street Capital Partners is a St. Louis-based private equity firm focused on investing in founder-led middle market businesses. TSCP has acquired more than 150 companies in the Healthcare & Life Science Services, Software & Technology Services and Business Services & Engineered Products sectors and have managed more than $2.6 billion since being founded in 2000. TSCP partners with management teams to increase value by accelerating growth, both organically and via complementary acquisitions.
International Consortium Awarded Eurostars Grant
International Consortium Awarded Eurostars Grant for the Development of First-in-class Treatment of Metastatic Cancer
ONA Therapeutics, Absolute Antibody and RIC partner to create new type of biologic
Barcelona, Spain, December 16, 2020 ONA Therapeutics, Absolute Antibody and RIC - three leading European companies in the biotech landscape - have been awarded a Eurostars grant for a two-year project that will develop a proprietary biological drug with a unique mode of action for the treatment of various types of metastatic cancers. Taking the lives of more than 9 million people globally each year, metastatic cancers are often unresponsive to existing cancer treatments, leaving a large unmet need. In order to tackle this important health problem, the newly established consortium - named LIPOLOGIC - will combine industry know-how in complementary areas of drug development: from target discovery, across molecule formatting and manufacturing, to lead selection and characterization.

US authorization of first COVID vaccine marks new phase in safety monitoring
The FDA has issued an emergency-use authorization for the Pfizer–BioNTech vaccine. Regulators are gearing up to look for side effects.

The US Food and Drug Administration (FDA) has granted an emergency-use authorization for the country’s first COVID-19 vaccine. The vaccine, made by Pfizer of New York City and BioNTech of Mainz, Germany, can now be administered in the United States outside a clinical trial.
The decision, announced on 11 December, trails behind emergency authorization of the same vaccine in the United Kingdom and Canada, and news that the United Arab Emirates has approved another COVID-19 vaccine, produced by Sinopharm of Beijing.
The FDA decision follows a vote by its advisory committee on 10 December: the committee voted 17 to 4, with one abstention, to recommend the vaccine.
Covid-19 vaccine from Pfizer and BioNTech is strongly effective
Covid-19 vaccine from Pfizer and BioNTech is strongly effective, early data from large trial indicate

Pfizer and partner BioNTech said Monday that their vaccine against Covid-19 was strongly effective, exceeding expectations with results that are likely to be met with cautious excitement —and relief —in the face of the global pandemic.
The vaccine is the first to be tested in the United States to generate late-stage data. The companies said an early analysis of the results showed that individuals who received two injections of the vaccine three weeks apart experienced more than 90% fewer cases of symptomatic Covid-19 than those who received a placebo. For months, researchers have cautioned that a vaccine that might only be 60% or 70% effective.
New research on SARS-CoV-2 virus 'survivability'
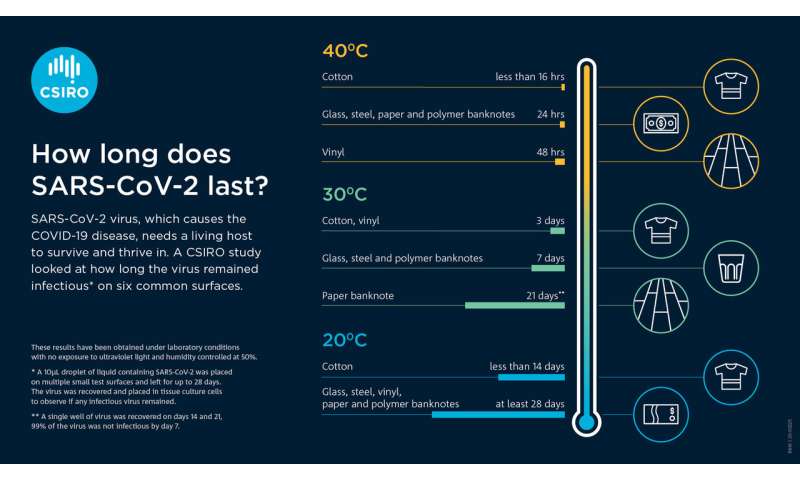
How long does SARS-CoV-2 last on different surfaces? Credit: CSIRO
Researchers at CSIRO, Australia's national science agency, have found that SARS-CoV-2, the virus responsible for COVID-19, can survive for up to 28 days on common surfaces including banknotes, glass—such as that found on mobile phone screens—and stainless steel.
The research, undertaken at the Australian Centre for Disease Preparedness (ACDP) in Geelong, found that SARS-CoV-2:
- survived longer at lower temperatures
- tended to survive longer on non-porous or smooth surfaces such as glass, stainless steel and vinyl, compared to porous complex surfaces such as cotton
- survived longer on paper banknotes than plastic banknotes.
Results from the study The effect of temperature on persistence of SARS-CoV-2 on common surfaces was published inVirology Journal.
Absolute Antibody Adds SeqPack™ Hybridoma Preservation Kit
Absolute Antibody Adds SeqPack™ Hybridoma Preservation Kitto Antibody Sequencing Service
Streamlines the sample submission process for hybridoma sequencing customers
Redcar, UK, September 15, 2020.Absolute Antibody Ltd., an industry-leading provider of recombinant antibody products and services, today announced the availability of the SeqPack™ Hybridoma Preservation Kit, which streamlines the sample submission process for hybridoma sequencing customers worldwide. The kit saves customers both time and money by eliminating the need to ship hybridoma cells on dry ice.

Moderna COVID-19 Vaccine Shows Immunogenicity in Older Patients
Moderna COVID-19 Vaccine Shows Immunogenicity in Older Patients
Moderna today presented Phase I data showing that its closely-watched messenger RNA (mRNA) COVID-19 vaccine candidate mRNA-1273 showed immunogenicity in patients 55 years old and older that was roughly the same or higher than data seen in younger patients at the dosage the company is using in its Phase III trial.
Moderna’s series of two 100 mcg doses of mRNA-1273 seroconverted all 120 participants in a Phase I trial (NCT04283461) after the first dose given on Day 1, with the area under the curve for all age groups exceeding the median of convalescent sera, Jacqueline M. Miller, MD, FAAP, Moderna’s senior VP of infectious disease development, stated during a presentation at the August meeting of the CDC Advisory Committee on Immunization Practices (ACIP).
0 Links
Absolute Antibody Offers SARS-CoV-2 Neutralizing Antibodies
Absolute Antibody Offers SARS-CoV-2
Neutralizing Antibodies Derived from COVID-19 Patients
Recombinant engineered antibodies available for coronavirus research and diagnostics
Redcar, UK, August 18, 2020.Absolute Antibody Ltd., an industry-leading provider of recombinant antibody products and services, today announced the availability of SARS-CoV-2 neutralizing antibodies derived from individuals infected with COVID-19. The antibodies, originally generated by Fred Hutchinson Cancer Research Center, have been engineered into recombinant formats useful for COVID-19 research and diagnostic development. They are now available to scientists and diagnostic developers worldwide via Absolute Antibody’s online catalog.

COVID-19 Research Methods: eBook Available
Whether you’re studying SARS-CoV-2 biology, developing therapies, or producing vaccines, fighting the COVID-19 pandemic requires an arsenal of specific research tools and methods.
The RayBiotech team has assembled a free, comprehensive guide to the technologies, reagents, and assays used in COVID-19 research. Click to get your copy.
Study suggests embryos could be susceptible to coronavirus
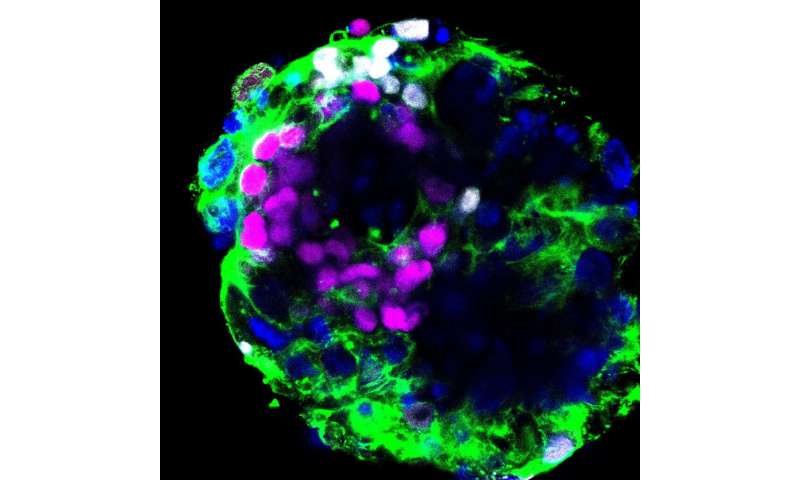
A human embryo cultured in vitro through the implantation stages and stained to reveal OCT4 transcription factor, magenta; GATA6 transcription factor, white; F-actin, green; and DNA, blue. Analysis of patterns of gene expression in such embryos reveals that ACE2, the receptor for the SARS-CoV-2 virus, and the TMPRSS2 protease that facilitates viral infection are expressed in these embryos, which represent the very early stages of pregnancy. Credit: Zernicka-Goetz lab
Genes that are thought to play a role in how the SARS-CoV-2 virus infects our cells have been found to be active in embryos as early as during the second week of pregnancy, say scientists at the University of Cambridge and the California Institute of Technology (Caltech). The researchers say this could mean embryos are susceptible to COVID-19 if the mother gets sick, potentially affecting the chances of a successful pregnancy.
Experts make weak recommendation for remdesivir in severe COVID-19
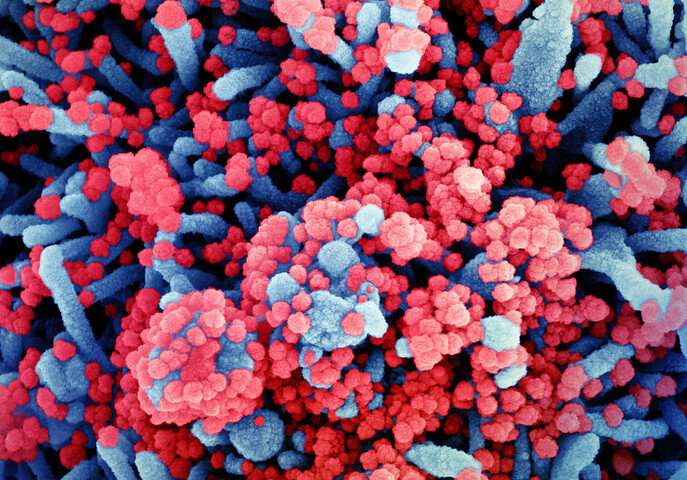
InThe BMJtoday, a panel of international experts make a weak recommendation for the use of remdesivir in patients with severe covid-19, and strongly support continued enrolment of patients into ongoing clinical trials of remdesivir.
Absolute Antibody and University of Zurich Collaborate
Absolute Antibody and University of Zurich Collaborate to Offer
Synthetic Nanobodies Against SARS-CoV-2 Receptor Binding Domain
Redcar, UK, July 8, 2020. Absolute Antibody Ltd., an industry-leading provider of recombinant antibody products and services, today announced a partnership with University of Zurich to offer synthetic nanobodies against the receptor binding domain (RBD) of SARS-CoV-2, the coronavirus that causes COVID-19. Under the partnership, the original nanobodies and newly engineered formats are now available to the global research community for use as serological controls and in COVID-19 therapeutic development. The synthetic nanobodies possess particular promise for the development of inhalable drugs, which could offer a convenient treatment option for the COVID-19 pandemic.
CRISPR-assisted novel method detects RNA-binding proteins in living cells

The yellow shade depicts the navigation system (CRISPR) that directs the CARPID components including BASU to the targeted RNA (a purple rope with stem loops on the left side). BASU "labels" (B) the adjacent binding proteins (RBP). Streptavidin (MyOne T1) is then used to recognize the binding proteins. Credit:Nature Methods(2020)
While scientists still don't fully understand the diverse nature of RNA molecules, it is believed that the proteins binding to them, called RNA-binding proteins, are associated with many types of disease formation. Research led by biomedical scientists from City University of Hong Kong (CityU) has led to a novel detection method, called CARPID, to identify binding proteins of specific RNAs in living cells. It is expected that the innovation can be applied in various types of cell research, from identifying biomarkers of cancer diagnosis to detecting potential drug targets for treating viral diseases.
Experimental peptide targets COVID-19
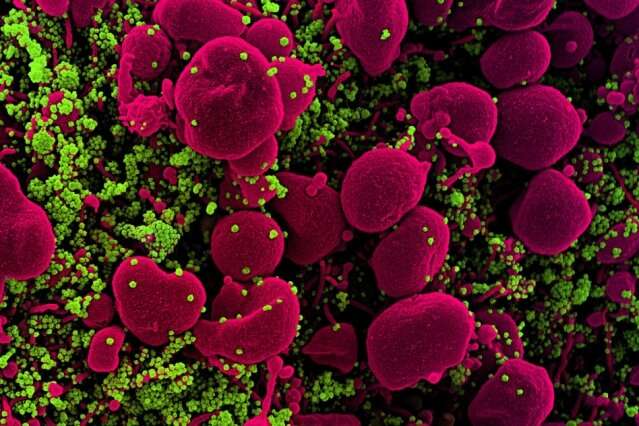
Colorized scanning electron micrograph of an apoptotic cell (pink) heavily infected with SARS-COV-2 virus particles (green). MIT researchers are using computational models of protein interactions to design a peptide that can bind to coronavirus proteins and shuttle them into a cellular pathway that breaks them down. Credit: National Institute of Allergy and Infectious Diseases/NIH
Using computational models of protein interactions, researchers at the MIT Media Lab and Center for Bits and Atoms have designed a peptide that can bind to coronavirus proteins and shuttle them into a cellular pathway that breaks them down.
Survival of coronavirus in different cities, on different surfaces
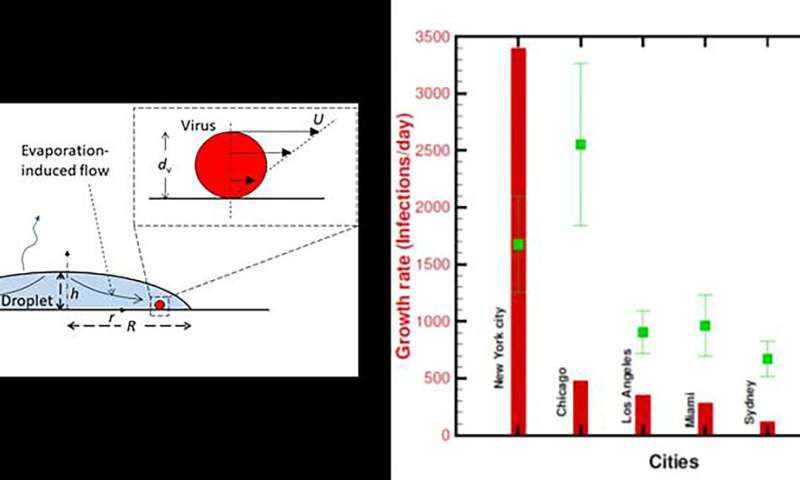
(left) A droplet on a surface. (right) Comparison of the growth rate of the infection of different cities/regions (bars) with respective drying times (squares) of a 5-nanoliter droplet. The error bar represents the variability in outdoor weather. Credit: Rajneesh Bhardwaj and Amit Agrawal
One of the many questions researchers have about COVID-19 is how long the coronavirus causing the disease remains alive after someone infected with it coughs or sneezes. Once the droplets carrying the virus evaporate, the residual virus dies quickly, so the survival and transmission of COVID-19 are directly impacted by how long the droplets remain intact.
Return to the lab: scientists face shiftwork, masks and distancing as coronavirus lockdowns ease
As scientists around the world return to work, they’re encountering new safety rules and awkward restrictions — and sometimes writing the protocols themselves
After her university closed in March, Jeannine Randall sat down to adapt her research plan for a pandemic. Her project to monitor tree swallows through the spring and summer with a team of three scientists would now require travelling to the nesting sites in separate vehicles, using individual work kits, staying 2 metres apart and, of course, sanitizing regularly. When she realized hand sanitizer was in short supply in shops, she made her own batch using ethanol from her lab.
Now, as her school, the University of Northern British Columbia in Prince George, Canada, resumes some services, she is putting the plan into action: counting eggs, waiting for hatchlings and watching the birds from daybreak to sundown. “I think scientists are very well placed in some ways to come up with a protocol that makes sense and then follow it,” says Randall, an avian ecologist.
Directed protein evolution with CRISPR-Cas9

With the help of CRISPR-Cas9, scientists can now develop proteins that make even difficult components of a mammalian cell visible under the microscope. Credit: MPI of Neurobiology/Arne Fabritius
"Directed evolution" is the process by which scientists produce tailor-made proteins for cell biology, physiology and biomedicine in the laboratory. Based on this method, Max Planck researchers from Martinsried have now developed a method to optimize proteins directly in mammalian cells. Using the new method, the scientists have produced the fluorescent protein mCRISPRred, which fluoresces brightly in lysosomes—very acidic, all-decomposing cell vesicles—which were previously difficult to label.
Nature-inspired CRISPR enzymes for expansive genome editing
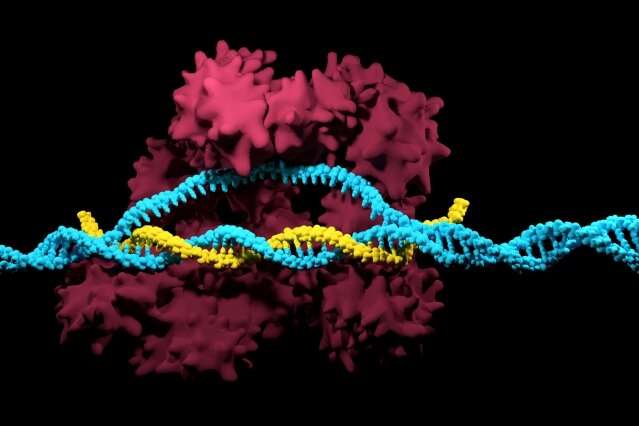
In nature, bacteria use CRISPR as an adaptive immune system to protect themselves against viruses. Over the past decade, scientists have been able to successfully build upon that natural phenomenon with the discovery of CRISPR proteins found in bacteria—the most widely used of which is the Cas9 enzyme. In combination with a guide RNA, Cas9 is able to target, cut, and degrade specific DNA sequences.
Video: When do the infected become infectious?
The danger posed by a person infected with COVID-19 doesn't begin the exact moment a few virus particles enter their system, so when do they start to be a health risk to those around them?
Penn State Professor Nita Bharti talks about incubation periods, latency periods, and what they mean for people who have been exposed.
Credit: Pennsylvania State University
Researchers crack COVID-19 genome signature
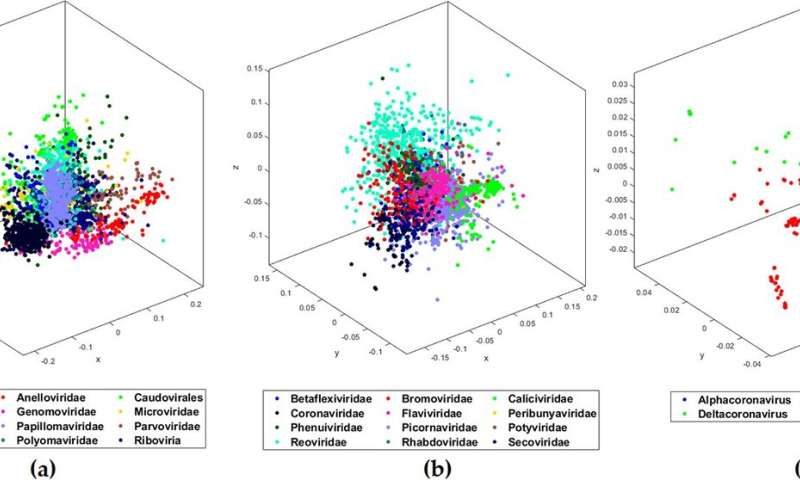
Using machine learning, a team of Western computer scientists and biologists have identified an underlying genomic signature for 29 different COVID-19 DNA sequences.
This new data discovery tool will allow researchers to quickly and easily classify a deadly virus like COVID-19 in just minutes—a process and pace of high importance for strategic planning and mobilizing medical needs during a pandemic.
Be proactive about mental health during COVID isolation, clinical psychologist says

Seeking out good news is a great way to keep mentally balanced during the long period of social isolation imposed by the COVID-19 battle, says a clinical psychologist who is an associate professor of psychology at The University of Alabama in Huntsville (UAH).
"I would like to urge everyone to look for the good news out there, especially the good news about COVID-19," says Dr. Eric Seemann. "Many valid news agencies have these stories on their websites but you need to look for them."
It may seem trivial but it's not, he says.
"Absorbing good news, such as the high recovery rate for most people who have contracted COVID-19, the promise of new treatments, and people-helping-people stories out there provide perspective and another frame of reference."
Finding leukemia's weakness using genome-wide CRISPR technology
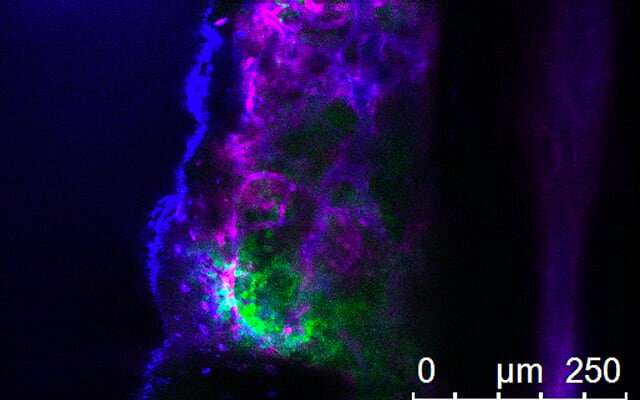
A team of researchers at University of California San Diego School of Medicine and Moores Cancer Center used CRISPR technology to identify key regulators of aggressive chronic myeloid leukemia, a type of cancer that remains difficult to treat and is marked by frequent relapse.
"We used CRISPR technology to carry out a genome-wide screen inleukemia cellsto block thousands of genes at once. This is an extremely powerful tool that allowed us to identify a multitude of genes that fuel leukemia growth and find new vulnerabilities that can be targeted in this disease," said senior author Tannishtha Reya, Ph.D., professor in the departments of Pharmacology and Medicine. "The study also shows, for the first time, that whole genome CRISPR-based screens can in fact be carried out in a manner that is much more physiologically relevant: using primary cancer cells, and in the setting of the native microenvironment."
New cancer drug shrinks tumors, reduces side effects, in animal studies
A class of experimental cancer drugs called BET inhibitors have shown promise for treating cancers of the blood, but can induce toxic side effects. Now Yale researchers have found a new inhibitor that in animal studies demonstrates greater potency against a wider variety of cancers, as well as against solid tumors, and also produces fewer side effects compared to others in the class.
The research was conducted by a team led by Qin Yan, associate professor of pathology and director of the epigenetics program, and appears in the April 14 online edition ofNature Communications.
Coronavirus: A map of Sars-CoV-2 activated proteins
Representation of the predicted SARS-CoV-2/Human interactome [26] (available for download at http://korkinlab.org/wuhanDataset), containing 200 unique interactions among 125 proteins (nodes). SARS-CoV-2 proteins are depicted as green circles, while human proteins are represented as squares. The color of human protein nodes reflects the integrated effect of MERS and SARS infections on the node network (see Supplementary Table S2) as a Normalized Enrichment Score (NES). Network visualization was performed via Cytoscape [49]. Credit: Courtesy of Journal of Clinical Medicine.
What happens when the pathogen responsible for the Covid-19 pandemic, the coronavirus Sars-CoV-2, makes contact with a human bronchial cell? A group of researchers from the Universities of Bologna and Catanzaro (Italy) mapped the interactions between the virus proteins and those of humans, showing which proteins are being "activated" and "de-activated" by Sars-CoV-2.
"Gaining knowledge about the molecular effects of Sars-CoV-2 on human proteins is fundamental to devise effective drug therapies," says Federico M. Giorgi, principal investigator of the study and a researcher at the University of Bologna. "Inhibiting the interactions that we mapped may represent an effective strategy for a therapy able to contain the disruptive force of Sars-CoV-2 and other coronaviruses onhuman cells."
An experimental peptide could block COVID-19
In hopes of developing a possible treatment for COVID-19, a team of MIT chemists has designed a drug candidate that they believe may block coronaviruses' ability to enter human cells. The potential drug is a short protein fragment, or peptide, that mimics a protein found on the surface of human cells.
The researchers have shown that their new peptide can bind to theviral proteinthat coronaviruses use to enter human cells, potentially disarming it.
World could face food crisis in wake of coronavirus: UN, WTO

The heads of three global agencies warned Wednesday of a potential worldwide food shortage if authorities fail to manage the ongoing coronavirus crisis properly.
Many governments around the world have put their populations on lockdown to slow the spread of the virus but that has resulted in severe slow-downs in international trade and food supply chains.
Connecting on Coronavirus: webinar series

The British Society for Immunology regarding our new webinar series: 'Connecting on Coronavirus: the expert hub'. The first webinar of the series is now open for registration and will take place this Thursday 2 April, 13:00-13:45 BST. Now more than ever, we need to keep up-to-date and stay connected within the immunology community, so we'd like to encourage you to book your free spot.
Interleukin-1β inhibition linked to reduced incidence of anemia
(HealthDay)—Inhibition of interleukin-1β (IL-1β) with canakinumab is associated with reduced incident anemia as well as improved hemoglobin levels among patients with baseline anemia, according to research published online March 24 in theAnnals of Internal Medicine.
Mounica Vallurupalli, M.D., from Brigham and Women's Hospital in Boston, and colleagues conducted an exploratory analysis of a multicenter randomized controlled trial. A total of 8,683 Canakinumab Anti-inflammatory Thrombosis Outcomes Study participants without anemia at trial entry and 1,303 with prevalent anemia at trial entry were randomly assigned to receive either placebo or canakinumab once every three months.
The researchers found that the incidence of anemia increased with rising baseline levels of high-sensitivity C-reactive protein (hsCRP); participants receiving canakinumab versus placebo had decreased hsCRP and IL-6. Participants without baseline anemia who received canakinumab had significantly less incident anemia than those who received placebo during a median follow-up of 3.7 years (hazard ratio, 0.84). The greatest benefits of canakinumab versus placebo on incident anemia were seen for participants with the most robust anti-inflammatory response. After two years of treatment, canakinumab increased mean hemoglobin levels by 11.3 g/L compared with placebo among those with baseline anemia.
Coronavirus lockdown: What I learnt when I shut my cancer lab in 48 hours
nature
Doing science during the quarantine in northern Italy has shown me that creativity needs connection. By Alberto Bardelli
I’ll never forget the e-mail I got at 12.49 p.m. on 9 March, telling me I had 48 hours to shut down my molecular oncology lab. I have a team of 24, including postdocs, PhD students, technicians, computational engineers and undergraduates. We study how colon cancer spreads and responds to therapy, using genomics and immunological approachesin vitroand in mouse models. The lab is part of acomprehensive cancer centrein northern Italy, which is experiencing a severe outbreak of coronavirus. The instructions from the institute’s scientific director were clear.Read More
Monkeys Develop Protective Antibodies to SARS-CoV-2
A small study of macaques finds they don’t develop a coronavirus infection the second time they are exposed, supporting the idea of using plasma from recovered patients as a treatment for COVID-19.
Whether people develop immunity to SARS-CoV-2 after being infected once is a pressing question for policymakers, public health professionals, and everyone affected by the spreading COVID-19 pandemic. It’s of particular interest to several research groups and companies currently developing plasma therapies, whereby antibody-containing blood plasma is extracted from recovered patients and administered to patients with severe cases to help them fight off the infection.
Now, a study in monkeys provides some clues. Three rhesus macaques did not develop a second infection after recovering from a first exposure to the coronavirus and being reexposed to SARS-CoV-2, suggesting that primates are capable of developing at least some short-term immunity to the pathogen. The research, posted as a preprint to bioRxiv March 14,has yet to undergo peer review. To the authors, the results indicate that reports of some COVID-19 survivors being “re-infected” a second time can be explained by issues with testing rather than a failure to develop immunity.
nature: A year without conferences? How the coronavirus pandemic could change research
As scientific meetings are cancelled worldwide, researchers are rethinking how they network — a move that some say is long overdue.
This is shaping up to be an unusual year — it might even be the year scientists stop going to conferences. As the coronavirus pandemic marches around the world, leading to unprecedented measures to stop the virus’s spread, the number of scientific conferences being cancelled is rising and researchers are scrambling to find alternative ways to share their work and interact with collaborators. Some of these discussions are even pushing researchers to rethink the concept of meetings entirely.
“At some point, we need to be having conversations about ‘What is the point of a conference now?’” says Sarah Hörst, a planetary scientist at Johns Hopkins University in Baltimore, Maryland. Although cultural changes happen slowly in the scientific world, she says, “I’m hoping this will at least force some real conversation.” Read More
Nature: Coronavirus latest: First vaccine clinical trials begin in United States
17 March 00:30GMT —First vaccine clinical trials begin in the United States
The first phase I clinical trial for a potential COVID-19 vaccine has begun in Seattle, Washington.
Four adults, the first of 45 eventual participants, received their first doses of an experimental vaccine developed through a partnership between the US National Institute of Allergy and Infectious Diseases (NIAID) andModerna, a biotechnology company based in Cambridge, Massachusetts. But although it is an important milestone, the phase I trial is just the beginning of a long process to test the drug’s safety and efficacy. Read More
PEPperCHIP® Coronavirus 2019-nCoV Proteome Microarry
In light of thecoronavirus disease (COVID-19) outbreak, PEPperPRINT has rapidly developed and produced a new peptide microarray based on the 2019-nCoV (Severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) viral genome. Derived from the virus isolate Wuhan-Hu-1 (GenBank ID: MN908947.3), the newPEPperCHIP®Coronavirus 2019-nCoV Proteome Microarray gives researchers access to serologically screen 4,883 individual peptides spanning the entire viral proteome. More here
Absolute Antibody Launches FleXpress™ High-Throughput Recombinant Antibody Production Service
Enables the rapid production of large numbers of recombinant antibodies at 80 ml scale
Redcar, UK, March 4, 2020. Absolute Antibody Ltd., an industry-leading provider of recombinant antibody products and services, today announced the launch of its FleXpress™ high-throughput recombinant antibody production service, which offers customers the flexibility to rapidly express a large number of antibodies at 80 ml scale. All production occurs in Absolute Antibody’s ISO-certified antibody manufacturing facility in Northeast England, which recently expanded to increase production capacity and meet the growing demand for recombinant antibody technology.
The high-throughput recombinant antibody production service is ideal for early-stage pharmaceutical researchers conducting pilot studies on a group of therapeutic antibody candidates, as well as reagent companies with a monoclonal antibody portfolio interested in converting their collection from hybridomas to recombinant production. Recombinant antibodies, long the standard in pharmaceutical development, are becoming increasingly popular for research and diagnostic applications due to their superior batch-to-batch reproducibility, antibody engineering options, and guaranteed long-term supply.
The antibody production service utilizes Absolute Antibody’s HEXpress™ antibody expression platform, a serum-free, mammalian transient expression system that offers a faster, more affordable alternative to stable CHO cell line generation. The proprietary system produces high-quality recombinant antibodies in any species, isotype or format, including multispecifics and fragments, with high purity and low endotoxin levels.
The FleXpress™ production line can recombinantly manufacture hundreds of different antibodies per week at 80 ml scale, allowing Absolute Antibody to quickly respond to customer needs for high-throughput small-to-medium-scale recombinant antibody production. It joins two other production lines at the company’s antibody manufacturing facility, which also offers the option to scale to multi-gram quantities in a matter of weeks.
“Absolute Antibody specializes in recombinant antibody technology, and we’ve developed a robust and reliable platform for high-throughput antibody production at relatively small scales,” said Catherine Bladen, PhD, Chief Operations Officer at Absolute Antibody. “Our new production line gives our customers the flexibility to rapidly and cost-effectively test recombinant versions of many different antibodies using our proven recombinant expression technology.”
In 2019, Absolute Antibody produced thousands of different recombinant antibodies, making 120 grams of protein in total, for customers across pharmaceuticals, biotechnology, research reagents, diagnostics, and academia.Since its founding in 2012, the company has also used its production platform to build a unique recombinant antibody catalog consisting of nearly 5,000 antibodies, all benefitting from antibody engineering technology. The FleXpress™ production service enables customers to utilize this same technology for their own recombinant antibody projects.
For more information, please visit our website here.
About Absolute Antibody, Ltd.
Absolute Antibody is a rapidly growing company with a vision to make recombinant antibody technology accessible to all. We offer antibody sequencing, engineering and recombinant production as custom services, as well as a unique catalog of recombinant antibodies, engineered into new and useful formats. Visit absoluteantibody.com for more information.
Knockout Cell Pools Accelerate Neurodegenerative Disease Modeling
Synthego strives to make CRISPR accessible and easy-to-use for researchers so that they can focus on discoveries rather than the method itself. In this mini case study, we discuss how Synthego’s knockout cell pools helped Dr. Eric Hoffman, Assistant Professor at the University of Pittsburgh, in developing cell culture models of neurodegenerative processes.
Listen to Synthego latest Crispr podcast here
How to determine the specificity of an antibody for Western Blot

The Western blot application combines the resolving power of polyacrylamide gel electrophoresis, the specificity of antibodies, and the sensitivity of enzyme assays. Althoughevery step of a Western blot procedure must be carefully considered, fundamental toits success is the ability of the primary antibody to detect the protein of interest. But how to validate an antibody for Western blot?
Absolute Antibody Partners with the Technical University of Denmark
Absolute Antibody Partners with the Technical University of Denmarkto Offer Antibodies against Animal Venom Toxins
Recombinant antibodies in newly engineered formats to further snakebite research and diagnostics
Redcar, UK, February 20, 2020. Absolute Antibody Ltd., an industry-leading provider of recombinant antibody products and services,today announced a partnership with the Technical University of Denmark to develop engineered recombinant antibodies specific for animal toxins, such as toxins from snake venoms. Antibodies targeting various snake and spider toxins are now commercially available in newly engineered formats, providing valuable research tools for scientists worldwide striving to produce better antivenom treatments.
More than five million people are bitten by snakes worldwide every year, resulting in 100,000 deaths, and many more amputations and other permanent disabilities. The only currently available treatment is antivenom derived from animal plasma, which is expensive and can result in serious adverse reactions. However, snakebite research has been hampered by limited attention and a lack of useful research tools. The World Health Organization (WHO) has therefore declared snakebite envenoming a neglected tropical disease to increase research toward better therapies and diagnostics.
The newly available toxin-specific antibodies are valuable tools for researchers studying snake venoms. Originally developed by the laboratory of Andreas Hougaard Laustsen at the Technical University of Denmark, the antibodies target cytotoxins and phospholipases A2from African cobras, latrotoxin from widow spiders, and myotoxin II from lancehead vipers.Under the partnership, Absolute Antibody has engineered the antibodies into new recombinant formatsand made them available to a wider scientific audience via an online reagents catalog.
The recombinant antibodies ensure batch-to-batch reproducibility while alsoopening up new experimental possibilities. For example, the antibodies are now available in a human format for use as serological controls, mouse and rabbit formats for detection applications, and sheep formats to enable better comparisons to traditional antivenom. Scientists can also request the antibodies in custom species, isotypes and subtypes best suited for their research project.
“We’re excited to partner with the Laustsen lab to offer recombinant anti-toxin antibodies,”said Dr. Michael Fiebig, Vice President Product Portfolio & Innovation at Absolute Antibody. “Our collaboration makes these valuable reagents more widely available to the research community and increases the breadth of possible applications through antibody engineering.”
“The importance of having useful and robust research reagents as a scientist cannot be overstated,” said Dr. Andreas Hougaard Laustsen, Associate Professor in the Department of Biotechnology and Biomedicine at the Technical University of Denmark. “I am very pleased to work with Absolute Antibody to jointly develop new toxin-specific antibodies, as increased access to these valuable tools will help toxinologists and antivenom researchers worldwide fight snakebite envenoming.”
For more information, and a full list of currently available antibodies, please visit our website here.
About Absolute Antibody, Ltd.
Absolute Antibody is a rapidly growing company with a vision to make recombinant antibody technology accessible to all. We offer antibody sequencing, engineering and recombinant production as custom services, as well as a unique catalog of recombinant antibodies, engineered into new and useful formats. Learn more here.
About Technical University of Denmark
DTU is recognized internationally as a leading university in the areas of technical and natural sciences, renowned for their business-oriented approach, focus on sustainability and study environment. The university was founded in 1829, and is now ranked as one of the foremost technical universities in Europe, continuing to set new records in the number of publications, and increase and develop partnerships with industry, and assignments accomplished by DTU’s scientific advice. Learn more here.
Information on the Laustsen Lab can be found here:https://tropicalpharmacology.com
Researchers identify new 'universal' target for antiviral treatment
As the coronavirus outbreak shows, viruses are a constant threat to humanity. Vaccines are regularly developed and deployed against specific viruses, but that process takes a lot of time, doesn't help everyone who needs protection, and still leaves people exposed to new outbreaks and new viruses.
Now, researchers at Massachusetts General Hospital (MGH) have uncovered a novel potential antiviral drug target that could lead to treatments protecting against a host of infectious diseases—creating a pan, or universal, treatment. Their work suggests that the protein Argonaute 4 (AGO4) is an "Achilles heel" for viruses.
AGO4 is one of a family of AGO proteins. Until now, there has been little evidence of why they are important. The researchers, led by Kate L. Jeffrey, Ph.D., and her collaborators found that AGO4 plays a key role protecting cells against viral infections.
Specifically, this protein is uniquely antiviral in mammalian immune cells. The group studied the anti-viral effects of several Argonaute proteins, and found that only cells that were deficient in AGO4 were "hyper-susceptible" to viral infection. In other words, low levels of AGO4 make mammalian cells more likely to become infected.
This study was published today byCell Reports.
The MGH researchers suggest that boosting levels of AGO4 could shore up the immune system to protect against multiple viruses. "The goal is to understand how our immune system works so we can create treatments that work against a range of viruses, rather than just vaccines against a particular one," says Jeffrey.
Mammals have four Argonaute proteins (1-4), which act by silencing genes and which are remarkably conserved throughout multiple living things, including plants. These are RNAi and microRNA effector proteins and RNAi is the major antiviral defense strategy in plants and invertebrates. Studies of influenza infected mice have shown that AGO4-deficient animals have significantly higher levels of the virus.
The next steps are to "determine how broad spectrum this is to any virus type," says Jeffrey. "Then we need to discover how to boost AGO4 to ramp up protection against viral infections."
More information: Fatemeh Adiliaghdam et al, A Requirement for Argonaute 4 in Mammalian Antiviral Defense, Cell Reports (2020). DOI: 10.1016/j.celrep.2020.01.021Journal information: Cell Reports
How to achieve greater separation in Western blot when proteins have a similar size

Despite its overall simplicity,the Western Blot applicationis a powerful procedure for the immunodetection of proteins and an essential tool within biological research. Nevertheless,proteins of the same length or similar size such as isoforms of the same protein, are difficult to separate.With some adjustments you can however achieve great results. Here we share some tips.
Control your IHC: primary antibody controls you should know

How many decisions do you think you make every day? Would you be surprised if I tell you that an adult person makes about 25,000 more or less conscious decisions every day? It’s a fact. Consciously or unconsciously, we spend every day of our lives making decisions: where to go, what to wear, what to eat, how to spend our time, etc. But how much time do you spend making a decision for the right control for your primary antibody? And, most importantly, did you make the right choice? How can you tell a good decision from a bad one?
Artifacts in IHC: the usual suspects - part II

“I don’t know why I am doing this. I can’t handle it!”You thought you knew how to recognize and solve your IHC artifacts but after reading the troubleshooting guide the answers seem so far away. Long lists of tips. Examples too difficult to understand. Not-so-friendly images. Feeling overwhelmed? You are not alone. Although sometimes troubleshooting seems unbearable, there is no need to worry! Troubleshooting is nothing more than the logical elimination of variables.
In our previous blog (Artifacts in IHC: the Usual Suspects - Part I) we have identified the major suspects (sample, antibodies and protocol) responsible for the most common IHC artifacts such as lack of staining, nonspecific binding and high background.
If you are now looking for tips on how to avoid artifacts in your IHC staining read on.
AR116
Artifacts in IHC: the usual suspects - part I

After a few intense days of meticulous tissue washes and antibody incubations my staining is finally ready. I am staring at it down the microscope. I enjoy what I see: a dark brown color exactly where I expect to see it! Am I good or what? I give myself a figurative pat on the back and an imaginary high five. Excited and proud, I call my supervisor who glares intently at it and then firmly, and rather surprisingly, asks me to re-run the staining. What went wrong? What did I miss? I must get to the bottom of this!
Tissue processing - how to succeed with your IHC part I

Would you print your childhood best and precious memories on a low-quality photographic film? I wouldn’t. However, just like me, you probably have hundreds of old faded yellow photos abandoned in a drawer. Images quality relies on high-quality printing films…and so does your immunohistochemical staining. Tissue handling and tissue processing determine the quality of your IHC results. You want high resolution? Let’s understand how to achieve it.
Absolute Antibody Launches VivopureX™ Recombinant Antibodies for In Vivo Research
Absolute Antibody Launches VivopureX™ Recombinant Antibodies forIn VivoResearch
Engineered antibodies available in bulk-discounted sizes improve research results in mouse models
Redcar, UK, January 7, 2020.Absolute Antibody Ltd., an industry-leading provider of recombinant antibody products and services, today announced the launch of its VivopureX™ recombinant mouse antibodies forin vivo research in mouse models. The collection consists of popular antibody clones, many originally obtained from rats or hamsters, which Absolute Antibody has engineered into mouse-anti-mouse recombinant versions to improve research results. The antibodies are all available in discounted bulk sizes ranging from 1 mg to 100 mg.
VivopureX™ antibodies are species-matched chimeric antibodies, consisting of a clone’s original antigen-binding variable domain with a mouse constant domain, which means they do not induce neutralizing antibodies in mouse models. As a result, the engineered recombinant antibodies offer many advantages compared to the original monoclonal antibodies, including increased long-term efficacy, stronger potency and a more consistent response across cohorts. In addition, VivopureX™ antibodies feature engineered effector functions, with Fc receptor binding tailored to best suit popular applications such as depletion, agonism or blocking.
All antibodies are produced recombinantly for ensured batch-to-batch reproducibility, and offer high purity and low endotoxin levels ideal forin vivoapplications. The antibodies are targeted against key immune system proteins, including clinically relevant checkpoint proteins such as PD-1, CTLA-4 and OX40. Absolute Antibody data has shown that the recombinant mouse PD-1 antibody, based on the widely used clone RMP1-14, reduces tumor size in a mouse model more effectively than the original rat version.
“The VivopureX™ antibody collection includes a selection of our most exciting mouse-anti-mouse antibodies, now available at discounted bulk prices ideal forin vivoresearchers,” said Dr. Michael Fiebig, Director of Products and Innovations at Absolute Antibody. “Most antibodies currently usedin vivoare immunogenic, leading to adverse immunological reactions and gradual loss of activity. By applying the same protein engineering approaches used in therapeutic development to research reagents, we can improvein vivoresearch results and further our understanding into the mechanisms underlying immunotherapy.”
For more information, and a full list of available antibody targets, please visit our website here.
About Absolute Antibody, Ltd.
Absolute Antibody is a rapidly growing company with a vision to make recombinant antibody technology accessible to all. We offer antibody sequencing, engineering and recombinant production as custom services, as well as a unique catalog of recombinant antibodies, engineered into new and useful formats. Visit absoluteantibody.com for more information.
Antigen retrieval - how to succeed with your IHC part II

How many samples? How much money and time have you wasted trying to unmask a protein on your IHC samples? Tissue fixation in immunohistochemistry often negatively impacts on your immunostaining by masking the epitope of interest. Read on to learn about the most common antigen retrieval methods used to restore epitope-antibody binding.
Interview with a scientist: IHC - past, present and future challenges

Today we meet with Dr. Caroline Kampf to ask her 10 questions about past, present, and future of immunohistochemistry (IHC).
In the last couple of decades, there has been an exponential increase in publications on immunohistochemistry technique spanning from cellular and molecular biology, biochemistry, pathology, histology, immunology, internal medicine, and surgery scientific articles. For its easy and rather inexpensive use in the search for tissue antigens that range from amino acids and proteins to infectious agents and specific cellular populations, immunohistochemistry is an essential tool in the diagnostic routine of pathological anatomy laboratories.
Absolute Antibody Partners with the Recombinant Antibody Network
Absolute Antibody Partners with the Recombinant Antibody Networkto Facilitate Access to Engineered Recombinant Antibodies
200+ recombinant antibodies to be engineered into new formats to advance research
Redcar, UK, December 3, 2019. Absolute Antibody Ltd , an industry-leading provider of recombinant antibody products and services, today announced a partnership with the Recombinant Antibody Network (RAN), a consortium of three expert centers at the University of Chicago, University of Toronto, and UC San Francisco (UCSF) with a common goal to generate recombinant antibodies at a proteome-wide scale. Under the new partnership, select recombinant antibodies made by the Recombinant Antibody Network will be engineered into new formats and made available to a wider scientific audience via Absolute Antibody’s online reagents catalog.
The sequences and validation data for more than 200 antibodies directed to intracellular targets generated by the Recombinant Antibody Network have been licensed to Absolute Antibody by the University of Chicago’s Polsky Center for Entrepreneurship and Innovation on behalf of the RAN. The collection includes antibodies against key transcription- and translation-regulating proteins, such as chromobox and bromodomain proteins, and other targets involved in important areas of biology research, including chromatin biology and infectious disease control. Absolute Antibody will use the sequences and data to recombinantly produce the antibodies in a variety of engineered formats designed to enable new experimental possibilities.
The engineered recombinant antibodies will be added to Absolute Antibody’s online reagents catalog, where researchers worldwide can easily access them. They will be offered in different species to readily enable co-labelling studies and in antibody fragment formats to reduce background in immunoprecipitation studies. All the antibodies are defined at the amino acid level to ensure batch-to-batch reproducibility, high purity and low endotoxin levels.
“We’re excited to partner with the Recombinant Antibody Network, which has been visionary in its efforts to generate a comprehensive set of recombinant antibodies for biomedical research,” said Amelia Gibson, PhD, MBA, director of business development at Absolute Antibody. “The Network shares our mission to further the availability of recombinant antibodies to advance scientific research, and we’re eager to bring this valuable collection of antibodies to a wider audience in newly engineered formats.”
To address the unmet need for an efficient pipeline for renewable antibody discovery, Anthony Kossiakoff, PhD, Otho S.A. Sprague professor of biochemistry and molecular biophysics at the University of Chicago, co-founded the RAN with two other veterans from the former Protein Engineering Department at Genentech Inc.Sachdev Sidhu, PhD, now a professor of molecular genetics at the University of Toronto, andJames Wells, PhD, professor of pharmaceutical chemistry in the UCSF School of Pharmacy, teamed with Kossiakoff to make automated, large-scale antibody production a reality. The RAN generates high quality non-animal derived recombinant antibodies from cloned synthetic genes that are selected for high performance.
“Our new partnership with Absolute Antibody significantly extends the reach of the recombinant antibodies developed at our three university sites,” said Kossiakoff. “Our consortium is committed to generating and validating functional antibodies for protein targets across the human proteome, and by partnering with Absolute Antibody, these antibodies will reach scientists worldwide for use in new applications, to further their contribution to scientific progress.”
Over the coming months, the recombinant antibodies will be added to Absolute Antibody’s reagents catalog here.
About Absolute Antibody, Ltd.
Absolute Antibody is a rapidly growing company with a vision to make recombinant antibody technology accessible to all. We offer antibody sequencing, engineering and recombinant production as custom services, as well as a unique catalog of recombinant antibodies, engineered into new and useful formats. Learn more at: www.absoluteantibody.com.
About the University of Chicago and the Polsky Center for Entrepreneurship and Innovation
As one of the world's premier research universities, the University of Chicago empowers students and scholars through its commitment to free and open inquiry. Across numerous departments and disciplines, as well as more than 140 institutes and centers, the UChicago community advances ideas and innovations that enrich human life. UChicago’s faculty are some of the top in the world. The University of Chicago has 89 Nobel Prize winners, including 6 current faculty, and receives more than $450 million in sponsored research awards each year. Learn more at: www.uchicago.edu.
The Polsky Center for Entrepreneurship and Innovation bridges the gap between knowledge and practice, idea and action, and research and impact through education, partnerships, and new venture creation. The Polsky Center represents the University of Chicago in its commercial transactions with the RAN and led the negotiation of the partnership with Absolute Antibody. Learn more at: polsky.uchicago.edu.
About UC San Francisco
The University of California, San Francisco (UCSF) is exclusively focused on the health sciences and is dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care.UCSF Health, whichserves as UCSF’s primary academic medical center, includestop-ranked specialty hospitalsandother clinical programs,and has affiliations throughout the Bay Area.Learn more at https://www.ucsf.edu, or see our Fact Sheet.
UCSF Innovation Ventures represents UCSF in its commercial transactions with the RAN.
About the University of Toronto
Founded in 1827, the University of Toronto is Canada’s leading institution of learning, discovery and knowledge creation. U of T is one of the world’s top research-intensive universities, driven to invent and innovate. It is also one of the top five universities in the world for its start-up incubator programs. In the last 10 years, the U of T entrepreneurship community has created over 500 companies and raised over $1.5 billion in investment capital. Learn more at: www.utoronto.ca.
About the Recombinant Antibody Network
The Recombinant Antibody Network (RAN) is a consortium of highly integrated technology centers at UCSF, the University of Chicago, and the University of Toronto, unified under a common goal to generate reliable high quality recombinant antibody (rAb) reagents at a proteome wide scale for biology and biomedicine. Learn more at: www.recombinant-antibodies.org.
BRD4: Epigenetic Regulator and Emerging Cancer Target

Bromodomain protein 4 (BRD4) is a transcriptional regulator that plays a key role in cancer, autoimmunity, and inflammatory diseases1,2. BRD4 was discovered as a protein bound to acetylated chromatin during cell cycle progression. In this way BRD4 maintains consistent gene expression during subsequent rounds of division, a phenomenon known as epigenetic memory or "bookmarking" for gene transcription2,7. BRD4 also plays a critical role in regulating differentiation and development2,4. In the absence of BRD4, bone marrow stem cells are unable to generate B and T cells2. BRD4 is required for the re-expression of stem cell genes during reprogramming of MEFs or B cells to induced pluripotent stem cells2, and also plays a role in osteoblast differentiation4. The role of BRD4 in cell cycle control and differentiation has made it an emerging therapeutic target for cancer and immune system pathologies.
Interview with a scientist: multiplex IHC - past, present and future challenges

Today we meet with Dr. Kristian Moller, Principal Scientist at Altas Antibodies, to ask him 10 questions about past, present, and future of multiplex immunohistochemistry.
Multiplex immunohistochemistry is a powerful investigative tool which allows for the simultaneous visualization ofmultiple proteins on a single section by using a specific antibody for each protein of interest.
Ushering in the Second Wave of Checkpoint Blockade Therapies
Contributed by Aliyah Weinstein, Ph.D.
Immune checkpoint blockade has emerged over the past decade as a useful therapeutic tool against many types of tumors. Checkpoint blockade therapies take advantage of the fact that many tumors are infiltrated by immune cells, but those cells are unable to effectively kill the tumor due to inhibitory signals that limit their function. By reversing inhibitory signals that block T cell functionality, checkpoint blockade therapy reactivates immune cells already in a tumor and allows them to resume their effector function1.
How can I stain multiple proteins in the same tissue or cell?

As a rule, the complex biology and spatial organization of tissues and solid tumors poses a scientific and diagnostic challenge that is not sufficiently well addressed by standard immunohistochemistry (IHC) procedures. How come this is the case? The answer is that single IHC staining provides data on only one marker at a time. So, how do you stain multiple protein in the same cell or tissue?
Which cells or organelles are my proteins expressed in?
An overview using the Cell Atlas

The human body is the results of trillion of cells communicating with each other. Early biologists described cells as simple membranous sacs containing fluid and a few floating particles. Today we know that cells are infinitely more complex than that. Read here about the Cell Atlas, part of the Human Protein Atlas, for insights into the spatio-temporal distribution of proteins within human cells.
Hydropac Insulated shipping systems
 Our Pharmapac range offers confidence and reassurance to Pharmaceutical, Biotech and Logistics companies, needing to transport temperature sensitive products; ultimately drugs or vaccines with a low thermal mass, for extended periods of time, whilst maintaining the desired cold chain.
Our Pharmapac range offers confidence and reassurance to Pharmaceutical, Biotech and Logistics companies, needing to transport temperature sensitive products; ultimately drugs or vaccines with a low thermal mass, for extended periods of time, whilst maintaining the desired cold chain.
3 steps to publishing a paper using the Tissue Atlas - a case study

In this blog, we would like to tell you about that time when researchers interested in a new therapeutic target for patients with type 2 diabetes, knocked at our door asking for help, and how we used the Tissue Atlas, part of the Human Protein Atlas project, to answer their questions. But let’s start from the beginning.
Every publication has its own unique story. Some are the result of serendipity, the accidental discovery of something good or useful while not specifically searching for it. The majority of publications are, instead, the result of a well-defined question or a clinical need such as the identification of targets suitable for drug intervention.
Genome-Wide Single-Cell Analysis with Antibody-Guided Chromatin Tagmentation Methods
Next-Gen ChIP-Seq: Genome-Wide Single-Cell Analysis with Antibody-Guided Chromatin Tagmentation Methods
New methods are improving the efficiency of epigenomic analysis, enabling analysis of histone modifications at the single-cell level. Many of these methods are variations on Active Motif’s patented TAM-ChIP™ technology, which utilizes antibody-guided tagmentation. Will these newer methods replace ChIP-Seq? What are the differences between the different protocols? Can you really investigate genome-wide histone modification profiles at the single-cell level? This article addresses these questions and more.
Can You Really Reverse Your Epigenetic Age?

A recent report in the field of epigenetics claimed that humans could reverse their epigenetic clock. This research led to both excitement that we might be able to take steps to live longer and healthier lives and skepticism that the results might not be real. This article covers all the details and separates the facts from the fiction.
The Pathology Atlas - a discovery journey in cancer research

Humans are an intrepid race. For centuries, explorers have embarked on ambitious journeys of discovery toward distant horizons in search of new lands and unfriendly shores. They were pioneers with limited resources, and powerful ideals. Today a new generation of visionary scientists sail to new frontiers and discovery. They sail with knowledge, strategy, timing and multiple resources to reach a destination and achieve a goal, seeking to develop newcure for human diseases, consciously engaging in development and innovation. Come aboard with me for this crusade to fight human cancer.
Learn how to use the Human Protein Atlas: part I - three ways to navigate the Tissue Atlas

Have you ever been lost? When you travel to a place that’s unknown to you, getting lost is easy, frustrating and discouraging – even frightening. And it’s always a waste of time. What you need is a map. A map shows detailed information about the landscape, roads, points of interest and distances. But just looking at the map it is not enough. First of all, you need to choose the correct type of map, be able to understand its features to be able to read it, and most importantly you need to find your location on the map! What if I tell you that there is a map of all the proteins in the human body? Would you be able to read it? This article is here to help you on your way to discovering the potential of this great tool.
Which tissues are my proteins expressed in? A guide to the Human Protein Atlas

Sitting at the lab desk in the late hour of my day, I navigate the web scrolling pages and pages of content to find the information I need to plan my next experiment: which tissue is my protein expressed in? I click dozens and dozens of links that take me from one page to another. I am confused. I am not a focused cyber-sailor and I usually get lost very easily only to find myself pages away from my true destination. Which tissue is my protein expressed in? I still have no answer. Frustrated, but happy to read on how to cook the best pizza in the world, I give up. Sounds familiar? Yes, it has probably happened to you as well.
Contribute to advancing science by publishing your negative results

We have run the exact same experiment. I found X. You found Y. Why? Have you been in this conversation with a collaborator? Have you ever obtained amazing results which contradict previous theory? Before you read on, take a moment to answer that for yourself. What was your reaction?“It is all my fault. I must have done something wrong”or “Interesting,I never thought about that before…”
Unexpected Results? If All Looks Well in the Experiment, Why Not Trust It?
Are you at times baffled or even disappointed about the unexpected outcome of your experiments? An unpredicted or negative result may be both frustrating and exciting at the same time. Maybe the scientific hypothesis addressed was wrong in the first place? It could also be due to inexperience, poorly planned experiments as well as faulty reagents. It could also be due to inadequate training, lacking scientific knowledge or poor experimental and/or analytical skills.
Can I use an antibody targeting a human protein in another model organism?

Can you make use of an antibody that targets a human protein in another model organism? Under the right conditions, the answer is yes!
Non-human model organisms are a vital tool in the discovery of the fundamental principles of human genetics and molecular biology. The majority of commercially available antibodies, however, are designed to bind human or relevant mammalian models, limiting the ability to perform comparative and translational studies using non-human/non-mammalian animal model organisms.
How do I know if I can trust my antibody?

Have you heard of The Domino Effect? I’m sure you have but let me remind you what it is. The Domino Effect is the cumulative, often disastrous, effect produced when one single event sets off a chain of other events, the last one depending on the very first one.
A ‘domino effect’ is not merely a phenomenon that just happens to you, but something you can control and drive. It is in your power to pick the cards that lead towards success. So why not to start with an antibody that you can trust?
Click here to find out more
Journals require validated antibodies. What does that mean for me?

Antibody validation is critical to reproducibility and a necessary step to increase the security of antibody specificity in every specific context relating the target protein, sample, and application. Yet all too often, antibody-based published data cannot be reproduced. If you want your scientific project to be taken seriously, you should spend some time upfront to validate your antibodies before starting the project. Read on to know why this is so important.
5 tips about validation when choosing an antibody

Say you’re in the lab, on your computer, searching for an antibody. After mining the data from the resulting loads of possibilities, your choice falls among 2 or 3 candidates. Great. But which one is the right one for you? Is it a complicated decision, or a simple one? Finding an antibody that works for your specific application can be a difficult task. Dozens of companies sell antibodies against your target proteins, and with so much choice available, looking for the perfect one can sometimes feel like a never-ending search. Read on. Here we share our 5 key points to ask yourself when choosing your next antibody.
Let’s say you made your choice and the newly purchased antibody arrives. Guess what? It doesn’t perform as advertised. This could be extremely frustrating especially when you’re under pressure to publish the study. The number one reason why an antibody doesn’t perform as promised is that it has not been properly validated, or not even tested, in the application you are using it. And now you are sitting there asking yourself: what do I do now?
To read more click here
Using RIME to Analyze Protein-Protein Interactions on Chromatin

Methods like chromatin immunoprecipitation and reduced representation bisulfite sequencing have made it easier for researchers to investigate epigenetic modifications on a genome-wide scale. However, one of the potential limitations of these methods is that you need to already have an idea about what epigenetic mechanisms are at play in your experimental system.
The ATAC-Seq method addresses this issue by giving researchers information about chromatin accessibility across the genome, regardless of the mechanism responsible. Many researchers have started using ATAC-Seq as a first-pass screening approach to identify changes in chromatin accessibility between samples, and then they can focus their follow-up experiments based on the results.
This article discusses what ATAC-Seq is, its history, how it works, and some discoveries enabled by ATAC-Seq.
Do you have your IHC controls under control?

Headphones on, I am listening to my favorite music and staring at the lab bench in front of me feeling excited to start the immunohistochemistry experiment of the day. Pipette, timer, wells, vials, brush, buffers. Protocol taped on the shelf. Everything is ready. I feel like a DJ at the console, and I find myself thinking: what makes a good DJ? A DJ does not just play one song after the other in a random sequence. He has everything under control, he plans and underpins, every minute of a performance to varying degrees. So, how do I get started when planning a new set for a forthcoming immunohistochemistry experiment?
Read on to find out how to control your results and prepare for a successful ’gig’ in the lab.
CRISPR enters its first human clinical trials
The gene editor targets cancer, blood disorders and blindness
Since its debut in 2012, CRISPR gene editing has held the promise of curing most of the over 6,000 known genetic diseases. Now it’s being put to the test.
In the first spate of clinical trials, scientists are using CRISPR/Cas9 to combat cancer and blood disorders in people. In these tests, researchers remove some of a person’s cells, edit the DNA and then inject the cells back in, now hopefully armed to fight disease. Researchers are also set to see how CRISPR/Cas9 works inside the human body. In an upcoming trial, people with an inherited blindness will have the molecular scissors injected into their eyes.
Those tests, if successful, could spur future trials for Duchenne muscular dystrophy, cystic fibrosis and a wide variety of other genetic diseases, affecting millions of people worldwide.
“CRISPR is so intriguing,” says Laurie Zoloth, a bioethicist at the University of Chicago Divinity School, “and so elegant.”
But big questions remain about whether CRISPR/Cas9 can live up to the hype. Other previously promising technologies have fallen short. For instance, stem cell injections helped paralyzed rats walk again. But they didn’t work so well for people, Zoloth says.
Complete Guide to Using RRBS for Genome-Wide DNA Methylation Analysis

DNA methylation is one of the most important epigenetic modifications because it contributes to the regulation of so many different biological processes and is involved in multiple different human diseases.
There are a lot of different ways to study DNA methylation, and the best method to use depends on the goals of the experiment. Many researchers want to identify the exact cytosine residues that are methylated in their samples and quantitatively know the levels of methylation at these sites in the different samples being compared.
One option for experiments like this is whole genome bisulfite sequencing (WGBS), which gives single-nucleotide resolution DNA methylation analysis across the entire genome. However, because the time and cost required for the sequencing and bioinformatics of whole genome assays are so high, many researchers are turning to a method called reduced representation bisulfite sequencing (RRBS) for their genome-wide DNA methylation assays.
Reduced representation bisulfite sequencing is a variation of whole genome bisulfite conversion sequencing that uses restriction enzyme digestion and DNA size selection to focus the analysis on a subset of the genome where the majority of the DNA methylation occurs. Focusing on this portion of the genome generates a genome-wide DNA methylation data set at a lower DNA sequencing cost than WGBS.
Read more about RRBS on the Active Motif blog.
Complete Guide to Understanding and Using ATAC-Seq

Methods like chromatin immunoprecipitation and reduced representation bisulfite sequencing have made it easier for researchers to investigate epigenetic modifications on a genome-wide scale. However, one of the potential limitations of these methods is that you need to already have an idea about what epigenetic mechanisms are at play in your experimental system.
The ATAC-Seq method addresses this issue by giving researchers information about chromatin accessibility across the genome, regardless of the mechanism responsible. Many researchers have started using ATAC-Seq as a first-pass screening approach to identify changes in chromatin accessibility between samples, and then they can focus their follow-up experiments based on the results.
This article discusses what ATAC-Seq is, its history, how it works, and some discoveries enabled by ATAC-Seq.
ChIP 101 eBook: A Beginner’s Guide to Performing Chromatin Immunoprecipitation Assays
Become a ChIP Assay Expert!
The chromatin immunoprecipitation (ChIP) assay is one of the best ways to study the association of DNA-binding proteins with chromatin and the localization of histone modifications throughout the genome.
But ChIP assays take time to master.
The free ChIP 101 eBook by Active Motif summarizes the key points that need to be paid attention to when performing ChIP experiments, helping beginners avoid common mistakes and master this technology more quickly.
Topics covered include:
- The 5 critical steps in every ChIP experiment
- How many cells are needed for ChIP assays?
- How to properly fix and prepare your samples for ChIP
- How to sonicate and solubilize chromatin
- The best type of antibody to use in ChIP assays
- The best ways to analyze ChIP results
Creative Biolabs’ DASS Has Unprecedentedly Accomplished 100% Accurate Distinction
Creative Biolabs’ DASS Has Unprecedentedly Accomplished 100% Accurate Distinction Between Leucine and Isoleucine
With more than 25 years of experience as a CRO company, Creative Biolabs has recently made a tremendous breakthrough in the field of de novo monoclonal antibody sequencing with the Database Assisted Shotgun Sequencing (DASS) technology. The DASS is advanced from the Next Generation Sequencing (NGS) platform, which was used to sequence the variable regions, variable plus leader regions, as well as the full-length heavy and light-chain antibodies of all species, isotypes, and allotypes.
Monoclonal antibody, a type of protein molecule, partakes an important role in the category of drug and diagnostic reagent. Its primary structure, especially the amino acid sequence of its Complementarity Determining Region (CDR), determines its biological function. Compared to the traditional methods, the DASS system can analyze the complete sequence of the monoclonal antibody much quicker and with higher accuracy, which brings hope to the medical research area.
Conventional methods for monoclonal antibody sequencing comprise Edman degradation, mass spectrometry, and traditional de novo sequencing.
1. Edman Degradation
Edman degradation is known as one of the classical methods that can obtain the exact sequence of the N-terminal mutant or modified proteins. However, this method is inefficient and single threaded when analyzing multiple proteins simultaneously. Moreover, Edman degradation reaction remains ineligible for the N-terminal blocked proteins, of which the alpha-amino is modified with acetylation, methylation, pyroglutamidation, etc. The antibody is first digested with protease at its N-terminal. Each of the resulting free alpha-amino acid is identified by High Performance Liquid Chromatography (HPLC). After several rounds of digestion and HPLC identification, the sequence at the N-terminal of the antibody then gets determined.
2. Traditional Mass Spectrometry
Traditional mass spectrometry was regarded as a comparatively comprehensive technology at the time, because it is high-throughput and cost-effective. Antibody sequencing mass spectrometry solved the principal problems encountered during the Edman degradation. Nevertheless, there still exist some deficiencies in this method. For example, users could receive false outcomes resulted from incomplete sequencing, omission of matching peptide segments with mass spectrometry, and mutation or unknown modification. Moreover, mass spectrometry is only effective when the sequence of the amino acids or protein database are available.
3. Traditional De Novo Sequencing
Evolved from the traditional mass spectrometry, the antibody de novo sequencing can solve most of the potential problems that arise from the above two methods. This method, integrated with the de novo algorithm, holds the capability of performing amino acid sequencing and post-translational modification analysis. It relies on the regular breakage of protease-cut peptide molecules in mass spectrometry detection. After finding the specific breakage mode, it can calculate the information according to the mass between peaks of mass spectrometry. The main technology involved is HPLC. However, this method is still limited in that it cannot precisely distinguish the difference of isobaric amino acids with exactly the same mass and very similar chemical characteristics.
Among the above-mentioned methods, the thorniest challenge rests with differentiating between leucine (Leu) and isoleucine (Ile). None of the methods could efficiently recognize the difference between the two amino acids with 100% accuracy. The randomly allocated Ile and Leu in the complementary determinant region fundamentally determines the antibody function. For example, some antibodies may not have any Leu or Ile in any of the 6 CDRs, while in some other cases there could be 9 in total. On average, a single CDR contains at least 3 to 5 Ile or Leu residues. It remains a problem for the researchers when sequencing antibodies and their functions. Results are usually imprecise since only the alignment method can infer their possible locations. Sequencing errors may cause significantly varied binding behavior. Even one misplaced amino acid in the primary sequence can destructively affect the final antibody structure and function. Thus, a strategy to distinguish Ile and Leu is urgently needed.
With accumulated experience, scientists from Creative Biolabs have pierced through the aforesaid problems and developed a Database Assisted Shotgun Sequencing (DASS) technology to precisely distinguish between leucine and isoleucine. The DASS is the first in the history of antibody sequencing to break through the problem faced with worldwide scientists working on the monoclonal antibody sequencing. The Ile and Leu in the CDR can now be accurately classified.
Monoclonal antibody sequencing plays a crucial role in enhancing antibody affinity and humanization through obtaining the first-order antibody sequence, which acts as the basis and prerequisite when developing antibody generic drugs. Direct sequencing analysis of existing antibody products on the market is a shortcut to developing antibody drugs, antibody diagnostic reagents and other antibody products. Hence, the advancement of precisely identifying the amino acids can significantly enhance the field of precision medicine. The DASS developed by the Creative Biolabs helps to build a solid function for subsequent application by saving time and economic costs, and improve the functional analysis and verification.
NEW product! Beta-amyloid 1-42 antibodies - HyTest
NEW product!
Alzheimer’s disease is a progressive neurodegenerative disease and the most common cause of dementia. To discriminate Alzheimer’s disease dementia cases from cognitively normal controls, the levels of beta-amyloid 1-42 (Aβ42) in the cerebrospinal fluid have been shown to have diagnostic significance. The concentration of Aβ42in CSF drops significantly 5-10 years prior to the establishment of cognitive impairment symptoms so it can be used as a biomarker at the earliest stages of the disease progression.
Aβ42 can be used for diagnostics of Alzheimer’s disease in both the prodromal and dementia stage of the disease, and it is included in the diagnostic research criteria for Alzheimer’s disease.
We provide threein vitroproduced monoclonal antibodies that are specific to human beta-amyloid 1-42. Correlation studies show good correlation between the prototype sandwich immunoassays and a commercially available test (INNOTEST BETA-AMYLOID (1-42) from Fujirebio, see Figure).
A Guide To Multiplexing - Atlas Antibodies
How can I stain multiple proteins in the same tissue or cell?
As a rule, the complex biology and spatial organization of tissues and solid tumors poses a scientific and diagnostic challenge that is not sufficiently well addressed by standard immunohistochemistry (IHC) procedures. How come this is the case? The answer is that single IHC staining provides data on only one marker at a time. So, how do you stain multiple protein in the same cell or tissue?
Immunohistochemistry (IHC) is the golden standard for the diagnosis of human diseases. In fact, to diagnose diseases like cancer, preclinical scientists and clinicians need to functionally profile individual cells within the tissues to grasp how diseases arise and progress. This, for example, helps to identify different subtypes of tumor cells and to tailor individualized therapies.
Conventional IHC is limited by spectral overlap which puts a restraint to the number ofcolors you can image in one section. For chromogenic detection this is usually limited to 5 dyes. Novel stable dyes offering other colors have increased the dynamic range of multiplexing. Iterated immunostaining involving cyclic removal of the primary reagent and repeated image capturing is commonly used, albeit time-consuming.
Visualizing multiple proteins in a single staining may beaccomplished by multiplex IHC.
Thermo Fisher Scientific Hosts Sixth Annual Applied Science Solutions Program
Encouraging and inspiring students in the primary and secondary grade levels to engage in science, technology, engineering and math activities is a core component of the community engagement strategy of Thermo Fisher Scientific. In 2018 alone the company hosted over 380 events around the World. Executing these activities involved almost 4,000 employees whose volunteer efforts impacted over 134,000 students. These students could become the STEM leaders of the near future. According to the U.S. Bureau of Labor Statistics, STEM jobs are set to grow to more than 9 million between 2012 and 2022.
Employees from the Thermo Fisher Scientific site in Logan, Utah, hosted their 6th annual Applied Science Solutions Program (ASSP). This eight-week challenge aims to provide students from fifth through eighth grade with practical opportunities to learn and apply STEM (science, technology, engineering and math) principles. More than 30 teams from three local school districts participated in this year's event.
Thermo Fisher , we have just updated our Antibody Toolbox
Introducing our new affinity matrix for purification of IgGs derived from rabbits. Learn More
Thermo Fisher Scientific ,start strong with the right protein gels
It's time for a new routine. Your western blots will be in better shape when you use protein gels with chemistries designed for your application. Whether your target protein is of high or low molecular weight, posttranslationally modified, or low or high in abundance, we have the right Invitrogen™ protein gel for you.
Introducing the new Antibody Resource Job Site
The world's most established antibody information site continues its development adding a new area to its highly successful platform of information to the biotechnology life sciences sectors
Since its launch in 1997, it's always evolved its offering to the market and has developed through product listings, advanced search facilities,content marketing plus direct & email promotional opportunities to its audience of focused industry contacts
The evolution continues as we are always looking for new ways to be of service to the marketplace. Our next advancement is designed to bring the quality of the Antibody Resource audience to the world of employment and you can click below
https://www.antibodyresource.com/jobsite/index.php
Promote your vacancy with Antibody Resource
We have created a new job platform where employers can appeal directly to our users to generate interest amongst and select from the best industry candidates.
3 Key Reasons to use Antibody Resource to fill your vacancies
1. A Responsive ,Quality Audience
Having the most established resource in this area ,you can trust that all the viewing audience are professionals and already in the industry. They are ready to consume information from the site and act accordingly once the right product or service is found.
The site ispositionedtogenerate interest from candidates with this key and qualified criteria,giving the company offering the role a heightened expectation of success
2.Covering the Market
This option is different to a standard job site as the potential candidates' are accessing the site for sector information to aid their working life. They have a keen interest in the market and a professional interest in organisations looking to hire
The advantage to this is that the site would be catching them at the ‘establishing the need' stage of the job seeking process. The next step of the 'Information search' can also be served asthe site offers the chance to to showcase the organisation and its employee culture through its 'Employer Profiles'
A posting on the Antibody Resource job board would then cover the whole market place of job seekers, at every stage, giving the organisation seeking to fill their role the most complete solution
3.Competitive Pricing Model
From our research across this market, we have looked to create the most cost effective option in the industry and if you are looking to fill your vacancies then is something that we are keen to help you with. Our pricing packages have been created to act as a very economical alternative to some of the more expensive options on the market, giving you a major incentive to see what the site can do for you
Please contact David Parker to start connecting with quality candidates and filling those industry roles
Tel : 07401 018191
Microbiome Therapeutics Europe
Microbiome Therapeutics Europe
28 - 29 March 2019
Courtyard by Marriott Vienna Prater/Messe, Vienna
lifesciences.knect365.com/microbiome-therapeutics-europe
On 28-29 March 2019, leaders from across the field of microbiome therapeutics will gathering in Vienna to discuss the latest research, developments, discoveries and data in the pursuit to translate the power of the human microbiome into commercially viable medicines.
Over 2 days, Microbiome Therapeutics Europe will explore the ins and outs of emerging technologies, learnings from clinical trials, regulatory and commercial challenges to help you gain the information and connections you need to take your pipeline to the next level.
Join the likes of AOBiome, AMC Amsterdam, Merck, Eligo Bioscience, The BioCollective, BioMarketing Insight, Evolve Biosystems Inc. and more for practical industry insights, and discuss manufacturing and formulation strategies to take these projects from concepts to market.
Plus, stay up-to-date of the regulatory framework developments and seek out investment opportunities to ensure you’re equipped with the information you need for your products to succeed.
Visit the website to see the full agenda and speaker line-up: https://goo.gl/4GGt3H
Book now: https://goo.gl/yuumCA
Complete Guide to Using Reduced Representation Bisulfite Sequencing (RRBS) for Genome-Wide DNA Methy
DNA methylation is one of the most important epigenetic modifications because it contributes to the regulation of so many different biological processes and is involved in multiple different human diseases.
There are a lot of different ways to study DNA methylation, and the best method to use depends on the goals of the experiment. Many researchers want to identify the exact cytosine residues that are methylated in their samples and quantitatively know the levels of methylation at these sites in the different samples being compared.
One option for experiments like this is whole genome bisulfite sequencing (WGBS), which gives single-nucleotide resolution DNA methylation analysis across the entire genome. However, because the time and cost required for the sequencing and bioinformatics of whole genome assays are so high, many researchers are turning to a method called reduced representation bisulfite sequencing (RRBS) for their genome-wide DNA methylation assays.
Reduced representation bisulfite sequencing is a variation of whole genome bisulfite conversion sequencing that uses restriction enzyme digestion and DNA size selection to focus the analysis on a subset of the genome where the majority of the DNA methylation occurs. Focusing on this portion of the genome generates a genome-wide DNA methylation data set at a lower DNA sequencing cost than WGBS.
Read more about RRBS on theActive Motif blog.
Everything you wanted to know about CRISPR but were afraid to ask!
CRISPR is one of the industry's hot topics at the moment and Novatein Bio have produced an overview of CRISPR,what it is and why it's so important.
Click below to understand its importance and why you need to know more
Can Targeted Epigenetic Changes Prevent Addiction?
There is growing evidence that alcohol and drug use can cause lasting genetic changes that may reinforce substance addiction that could also be passed down to future generations. Epigenetics focuses on how environmental factors can cause immediate genetic changes. DNA methylation is one epigenetic mechanism that is affected by external factors including environmental stress, chemical exposure, and disease. Various substance abuse and disorders have been linked with behavioral changes that are associated with increased DNA methylation.
To read more on this important area then click below and get more information from Enzo Life Sciences and their range of products for Epigenetics Research
Multiplex Single-Molecule Resolution Epigenetic Analysis with ChIA-Drop & More Epigenetics News
The epigenetics field is moving quickly, hundreds of new papers are published every month, making it hard to keep up with the newest findings. We’re trying to make it a little easier for you to stay up-to-date in this exciting area by scouring the literature and bringing you short and easily digestible summaries of the most interesting and most impactful discoveries in epigenetics research each month.
This month we cover ChIA-Drop, a new method for investigating chromatin interactions with single-molecule precision, a role for DNA methylation in coronary artery disease, and an epigenomic study that identified DNA sequence motifs associated with histone modifications in both human and mouse cells.
GeneTex Partners With BenchSci to Augment Antibody Catalog Publication Data
Partnership will use BenchSci's artificial intelligence to help researchers run successful experiments with the right antibody
Irvine, California, November 19, 2018 (Newswire.com)- GeneTex, Inc., a research antibody manufacturer, and BenchSci, a life science machine learning startup backed by Google's AI fund, have announced a new partnership to overcome a key challenge that biomedical researchers face when selecting antibody reagents for their experiments.
It is well documented that research antibodies lacking specificity and reliability have resulted in staggering amounts of wasted money, time, and effort, not to mention questionable scientific conclusions. This severely compromises the pace of discovery and slows productivity, with particularly negative implications for progress against disease and other major challenges impacting our society.
To prevent this, researchers review scientific papers for published data on antibodies when planning experiments. However, more than 38 million scientific papers have been published since 1980, a number that doubles every nine years. It can require hours, and sometimes days, to find useful data —if it can be found at all.
"GeneTex and BenchSci share the same goal, to put the best-suited antibody in the hands of the researcher."
- ALLEN LEE
VICE PRESIDENT
GeneTex's partnership with BenchSci aims to solve this problem by combining the company's ~50,000-reagent catalog with BenchSci's industry-leading artificial intelligence platform. BenchSci will use machine learning to identify published data for individual GeneTex products in openand closed-access datasets, display related published figures, and allow researchers to search using important experimental variables.
Allen Lee, Vice President of Business Development at GeneTex, believes that this relationship with BenchSci represents another component of his company's constant effort to offer better validation of its antibody products and to assist scientists in their selection of the best reagent for a given experimental application. "GeneTex and BenchSci share the same goal," he notes, "to put the bestsuited antibody in the hands of the researcher."
Liran Belenzon, CEO, BenchSci, says that the partnership will increase discoverability of GeneTex products in more than 1,300 academic institutions and 14 top pharmaceutical companies where researchers rely on BenchSci for their antibody searches. "BenchSci has proven to help researchers find the right antibodies up to 24x faster," he says. "With our GeneTex partnership, researchers can now also purchase those antibodies faster, further speeding research to drive more rapid discoveries."
To found out more about GeneTex and BenchSci visit them at the links below
http://www.genetex.com/
https://www.benchsci.com/
Biotech start-up becomes multi-award winner
This autumn is rich in awards for biotech start-up company C4Diagnostics, which is currently developing a breakthrough technology for in vitro diagnosis of infectious diseases based on patents from the CNRS and the University of Aix-Marseille.
After its first international recognition in the Universal Biotech Innovation 2017 competition and its integration into the accelerator Cisbio group, a leading player in in vitro diagnostics and life sciences, today C4Diagnostics announces that it has won the Invest Award of INNOV'inMed 2017, awarded by a jury of investment funds and innovation professionals. These numerous announcements come as the company is in the process of raising funds and is in discussions with various associations of business angels as well as French and international investment funds.
"This latest award is very exciting for us, and is a recognition of the work of the team and of our partners and advisors, who have accompanied us through the different stages from the beginning of the company. It is extremely encouraging for the next steps in our development" says Younes Lazrak, President of C4Diagnostics.
You can read more here https://www.cisbio.com/drug-discovery/c4diagnostics-awarded-its-innovation-0
Proteintech Group opens $1000 Best Lab Manager Award for nominations
Proteintech Group, the benchmark in antibodies, is inviting researchers from the US and Europe to nominate an outstanding lab manager for their 2018 Best Lab Manager Award.
Presented annually, The Best Lab Manager award aims to recognise those lab managers who go above and beyond the call of duty, rewarding the winner with a $1000 grant to support the research projects within their lab and a care package (Award plaque, commemorative mug and $100 Visa gift card).
Proteintech believes recognition in the lab is key. Honouring the unsung heroes keeping the lab in check is important for both lab morale and productivity. “The lab can be a chaotic place” says Dr William Olds, Proteintech Scientific Officer. “Bench work is only part of a successful lab. Staying up-to-date with regulatory compliances, day-to-day budgeting, ordering, training, and more are key to sustaining quality work long term. These roles often fall onto lab managers and success in these roles does not equate to authorship on a publication, unfortunately. We want to celebrate lab managers for creating and maintaining an environment that fosters breakthrough discoveries.” – Dr William Olds, Proteintech Scientific Officer.
The award is the 3rd annual Best Lab Manager award offered by Proteintech. Previous winners of the award include Dr Mary Skinner, University of Michigan USA and Dr Clare McManus University of Manchester UK who were each awarded $1000 for their outstanding work and dedication to the lab.
Read more about the award and previous winners here.
How to nominate a lab manager for the award
To nominate a lab manager for the award researchers must submit a 250-word summary explaining why they believe the lab manager deserves the award. 5 outstanding candidates will then be selected by the Proteintech judging panel based upon their selflessness, teaching ability, and project management skills.
Nominees and lab managers must be based in a research laboratory in a university or research institution to be eligible for the award. The deadline for nominations is November 8 and the winner will be announced on December 3.
Rockland Announces General Release of BioQuantiPro™ Host Cell Protein Screening Kit
Rockland Immunochemicals, Inc. announced today the general release of their new BioQuantiPro™ CHO-HCP ELISA Kit for detection and screening of host cell protein (HCP) contaminants in bioprocessing.
The kit was specifically designed with Rockland’s novel, validated antibodies to detect HCP contaminants and to measure analytes from crude cellular harvests, cell culture lysates, and process intermediates. The BioQuantiPro CHO-HCP ELISA Kit boasts the highest coverage of generic CHO-HCP kits, low variability, and, most importantly, the data to back it all up.
“While working with bioprocessing clients, we consistently hear the same complaint about HCP kits: ‘The generic kit I am using works OK, but I wish I had reliable data that I can trust for the validation of the antibodies used in the kit’” commented Karin Abarca-Heidemann, PhD., Vice President of Science Operations. “We aimed to create a kit that fills the void by providing transparency into the data necessary to develop accurate bioprocessing standards with confidence. We are proud of what we have accomplished with this new kit and look forward to working with customers to advance the science of bioprocessing.”
Rockland has a long history of developing process-specific HCP antibodies in the biotherapeutics field. “Over the last 15 years we have successfully developed and manufactured custom processspecific HCP antibodies for our customers.
In the design and function of this CHO-HCP kit, we drew upon our experience gained from working with a diverse set of biopharmaceutical clients. We are eager to see the feedback of the scientists who need this kit,” said David Chimento, PhD., Vice President of Client Solutions.
The BioQuantiPro CHO-HCP ELISA Kit is the first of several bioprocessing kits Rockland expects to release over the next eighteen months. Expect more and get more from Rockland in forthcoming kits for HEK 293 and Escherichia coli expression systems and other common bioprocessing contaminant targets.
Rockland Immunochemicals, Inc. CSO opines “Benefits of Polyclonal Antibodies”
Limerick, PA. August 9, 2018 – Rockland Immunochemicals, Inc. announced today that Dr. Carl Ascoli, Chief Science Officer at Rockland, and Dr. Birte Aggeler, Director of Antibody Development at Bio-Techne, composed a peer-reviewed article using their combined experience of more than 50 years. The review article was published online on August 9, 2018 and will be printed in the September 2018 issue by the journal BioTechniques. The review article, entitled “The Overlooked Benefits of Polyclonal Antibodies,” discusses the pros and cons of polyclonal, conventional monoclonal, and recombinant monoclonal antibodies, while presenting procedures for experimental design, the inclusion of relevant controls, and validation strategies for polyclonal antibodies.
Antibodies are critical reagents most often used by life science and translational medicine researchers and are transformative tools used to diagnose and treat disease. Many 21st century medical breakthroughs were successful in part to antibody technology. Yet antibodies, especially polyclonal antibodies, are caught in a firestorm of controversary concerning data reproducibility. Ascoli stated “by emphasizing the appropriate role polyclonal antibodies may play in conducting life science research, our efforts here should improve upon the use of antibodies, the collection of reproducible data, and the expansion of antibody-based technologies.”
Thoughtful antibody design and development allows for polyclonal antibodies to be used in many different applications, techniques, and instrumentation. Ascoli explained that “while some have called for polyclonal antibodies to be phased out of research entirely, we believe in using ‘the right tool for the job’ which includes using all forms of antibodies, polyclonal, conventional monclonal, and recombinant monoclonal, in the appropriate context and according to the manufacturer’s recommendations.” When using polyclonal antibodies, it is particularly important to use appropriate positive and negative controls and to assure immunoassay-specific antibody validation on a lot-to-lot basis. This review article presents the value of polyclonal antibodies for research, discusses strategies to minimize their disadvantages, and suggests when other forms of antibodies are more appropriate.
Bio X Cell is excited to introduce a new anti-mouse VISTA monoclonal antibody!
This antibody is useful for:
- in vivoandin vitroblocking ofVISTAsignaling
- IdentifyingVISTAexpressing cells via flow cytometry
Why isVISTAsignaling important?
VISTAis a recently discovered immune checkpoint proteinfound at high levels on myeloid cells that infiltrated tumors. Preclinical studies withVISTAblocking antibodies have shown promising improvements in anti-tumor T-cell responses. As always Bio X Cell antibodies are specifically formulated forin vivouse and feature:
- > 95% purity
- Ultra-low endotoxin levels
- Preservative, stabilizer, and carrier protein-free
Half Price on Immunostar’s heavily validated GLP2R primary antibody
Purchase Immunostar’s primary antibody, GLP2R (Glucagon-Like Peptide 2 Receptor) (#24200), on or before July 31st 2018 and receive 50% off. Use code, “AR50” at checkout.
GLP2R is an emerging neurotransmitter involved in feeding control inthe hypothalamus and brain stem. ImmunoStar’s GLP2R Antibody ensures
highly specific staining of neurons in the hypothalamus, brain stem,pancreatic α-cells, enteroendocrine cells in the intestine, as well as
enteric neurons, and vagal sensory neurons.
Rockland Immunochemicals, Inc. CSO Testifies on Scientific Reproducibility Issues
Limerick, PA. April 18, 2018 – Rockland Immunochemicals, Inc. announced today that Chief Science Officer, Dr. Carl Ascoli testified at the National Academies of Sciences, Engineering and Medicine’s third of six public session on Reproducibility and Replicability in Science on April 18th.
The National Academies are charged by Congressional mandate to explore the issues of reproducibility and replication in scientific and engineering research. The committee, composed of 15 subject matter experts, will assess what is known and identify areas that need more information regarding issues of replication and reproducibility; consider if the lack of replication and reproducibility impacts the health of science and the public’s perception; and draw conclusions and make recommendations to Congress that may have consequences affecting levels of government funding.
The open session portion of the April 18th meeting featured speakers from various disciplines including physics, engineering, life sciences, statistical analysis and economics. Dr. Ascoli presented to the committee during the Industry Perspective panel session. Dr. Ascoli stated, “While data irreproducibility of life science research tools has been reported to be caused by faults in study design, biological reagents, protocols, data analysis and reporting, a disproportionate amount of media attention has focused on biological reagents including cell lines, antibodies and animal models.”
Ascoli went on to explain that Rockland and other leading antibody manufacturers and resellers, along with funding agencies and some journals, are embracing change that will make a difference.
For instance: (1) better use of standards and controls can be made when collecting experimental data; (2) the ‘knowledge gap” that has resulted from outsourcing antibody technology can be addressed; (3) funding agencies can require precise description of reagents and methods to foster reproducibility; (4) journals can publish all necessary details of reagents and protocols critical for reproducibility; and (5) antibody manufacturers and resellers can adopt immunoassay specific validation guidelines and provide greater transparency when describing antibody production, screening methods and release criteria.
Opinion leaders testifying at this session challenged whether a “crisis” of reproducibility exists, as some have claimed in the literature, especially when considering that the clear majority of research performed results in the collection of high quality data sufficient to support and reproduce scientific claims. For instance, the pace at which new treatments have accelerated from the bench to the clinic has resulted in an explosion of breakthroughs in medicine, and antibodies are central to the discovery, diagnosis and cure of many diseases that just a decade ago were considered to have poor patient outcomes.
The root causes of data irreproducibility identified at this meeting were biases in experimental design, biases in statistical analysis, the resultant inaccuracies of mathematical standards and constants, and the inherent variability that exists when biological reagents are used. Focus on these causative factors is imperative as the stakes are high. One economist speculated that the potential economic impact of data irreproducibility, especially for pre-clinical and clinical applications, ranges from 190 to 380 billion US dollars, cumulatively.
The committee was given a timeline to assess issues pertaining to reproducibility and replication, hear testimony by subject matter experts, deliberate and complete their investigations within approximately 18 months of the start of activities in October 2017. At the conclusion, a written report will be issued to Congress that includes an assessment of current activities to improve reproducibility and replication highlighting examples of good practices and examine factors that adversely affect reproducibility and replication.
Buy a Primary Antibody and get a Secondary Free from Boster
Get one secondary antibody for free (biotin or HRP conjugated) with each purchase of any primary antibody! All other secondary antibodies, including fluorescence conjugated and SABC kits, will be 50% off when purchased with any primary. Boster will match each primary antibody you order with a conjugated secondary validated for WB, IHC, Flow cytometry, and more. This promotion is applicable to all countries.
ABclonal are offering 40% of all their Epigenetics Antibodies
There are over 1400+ Antibodies and the offer is running to May 31st 2018.
Are you using the terms IHC, ICC, & IF correctly? Find out how most scientists are not
Thermo Fisher Scientific ,Visit us at booth F35 in the Laboratory Hall
Join ThermoFisher Scientific at BPI Europe in Amsterdam
We are pleased to announce that Thermo Fisher Scientific will be present at the upcoming BioProcess International European Summit, April 23–25, Amsterdam, The Netherlands.
We look forward to welcoming you at booth #34.
Commercial Director to attend AACR 2018
Jonathan Baron, our Commercial Director will be attending AACR 2018 in Chicago from April 15th to April 18th.
We are always happy to meet the researchers and suppliers to find out how we can develop and improve Antibody Resource.
If you would like to arrange a meeting or just have an informal conversation, please e-mail antibody@antibodyresource.com
Learn about HTRF from Cisbio
2x cheaper and 3x faster than ELISA
White paper on Enhanced Antibody Validation from Atlas Antibodies
Enhanced Validation of Antibodiesprovides an overview of how antibodies are characterized and validated at Atlas Antibodies. Here you can learn aboutenhanced validation of antibodies,and well as how enhanced validationoffers an additionallayer of security whenusing our antibodies.
White paper on Immunohistochemistry in Human Tissues from Atlas Antibodies
Immunohistochemistry in Human Tissuespresents the immuhistochemistry application, and how the use of tissue microarrays has allowed for large scale protein expression profiling within the Human Protein Atlas project.
Absolute Antibody & Kerafast Merge
Merger brings together Oxford, UK and Boston, MA companies offering cutting-edge research tools
February 12, 2018. Absolute Antibody Ltd., an Oxford, UK-based company with a vision to make recombinant antibody technology accessible to all, and Kerafast Inc., a Boston, MA-based company with a mission to facilitate access to unique laboratory-made reagents, today announced a merger of the two companies. The merger brings together two companies with a shared commitment to improving the selection of research tools available to the scientific community.
Absolute Antibody specializes in the sequencing, engineering and recombinant production of antibodies. Their catalog offers more than 3,400 recombinant antibodies in new engineered versions, absolutely defined at the amino acid level to ensure batch-to-batch reproducibility. Their custom expression and engineering services have been used by 14 of the top 15 pharmaceutical companies by R&D spend, as well as groups from biotechnology, diagnostics and academia, for projects such as antibody sequencing, chimerization, humanization and more.
Kerafast has been growing a catalog of more than 3,500 unique reagents – including antibodies, cell lines, proteins and more – from prominent research institutions worldwide. The company has established partnerships with 159 institutions to facilitate access to materials developed by their investigators, a model which helps remove traditional barriers to transferring biomaterials among scientists.
The combined company will have offices in both the United States and United Kingdom, enabling a larger global reach, more efficient fulfillment of international orders, and better support for custom service customers. The staff in both locations will work together to further availability of recombinant antibodies and other novel research tools, in particular by licensing antibodies that would be good candidates for recombinant production.
“We are very excited to be joining forces with the Kerafast team,” said Dr. Nicholas Hutchings, founder and CEO of Absolute Antibody. “We believe all researchers should have access to recombinant versions of antibodies, especially those developed in academic laboratories that have been the bedrock of so much scientific research. Kerafast’s strong existing relationships with research institutions will allow us to advance the creation and availability of recombinant antibodies.”
“We are delighted to merge with Absolute Antibody, whose team shares our dedication to helping researchers harness the power of reagents to accelerate scientific progress,” said Dr. Jennifer Rossi, Chief Operating Officer of Kerafast. “Together, our staff will continue to change the way unique and useful research tools are created, accessed and used by scientists worldwide.”
For now, Absolute Antibody and Kerafast will retain their independent names, websites and employees under one umbrella company and will be working to integrate operations over the coming months.
Thermo Fisher Scientific Wins CiteAb 2018 Award for its Antibody Validation Initiative
Thermo Fisher Scientific has been named the winner of the 2018 CiteAb Award in recognition of the company’s Antibody Validation Initiative, a two-part testing method that is applied across its most widely used Invitrogen primary and secondary antibodies for specificity and utility in different research applications.
“Our judges for this category were impressed by the thoroughness of Thermo Fisher Scientific using so many methods to address specificity, but also of its novelty, making it easy to understand for customers and therefore easy to navigate to validated reagents on the company’s website,” said Dr. Andrew Chalmers, CiteAb co-founder.
Thermo Fisher’s two-part testing approach uses one of nine specificity tests, including: IP-mass spectrometry, knock out, knock down, independent antibody verification, cell treatment, relative expression, neutralization, peptide array and an orthogonal method. By applying a variety of specificity testing methods, researchers can remain confident that the appropriate validation process has been applied based on the use and function of a specific antibody.
Upon completion of the comprehensive testing method, each antibody receives a “badge” on the company’s website, called the Advanced Verification Badge. This label provides customers with further assurance of its performance and use in their experiments. To view an example, visit www.thermofisher.com/p53primaryab
Founded by Dr. Chalmers, Senior Lecturer, University of Bath in the United Kingdom, and Dave Kelly, Managing Director of Storm Consultancy, CiteAb uses the number of citations as a transparent method to rank antibodies.
To learn more and see examples of Thermo Fisher’s antibody validation, click here
Cite your research and receive a 10% discount
"Citing your sources correctly can dramatically speed up discovery for your fellow scientists."
Abbexa is offering those that share detailed publications a complimentary 10% discount on their next purchase with us. Simply purchase and cite our products in a publication. The sharing of scientific research publications is as important as cooperation and communication, and is one of the biggest contributors to achievement. This is why Abbexa is supporting and promoting publications and complete citations with this offer.
How to receive your discount
 Specify Abbexa as the source of the product
Specify Abbexa as the source of the product
 Include the unique product abx catalogue number
Include the unique product abx catalogue number
 Report the tested applications, dilution ranges and the species of the samples used
Report the tested applications, dilution ranges and the species of the samples used
 Email us a PDF version of your paper to info@abbexa.com
Email us a PDF version of your paper to info@abbexa.com
 Receive your unique discount code
Receive your unique discount code
ICI Boston Summit
March 19-21, Boston MA
Website: ICI Boston Summit
ICI Boston 2018 is focused exclusively on overcoming the preclinical, translational and clinical challenges encountered in the development of checkpoint modulators. Ensure you make the right decisions with your IO pipeline by learning from the drug developers who have successfully developed pioneering IO therapeutics.
Visit the website to learn more about the data and insights the 30+ expert speakers will share: ICI Boston Summit
Ensure the success of your research with CST
For Research Use Only, Not For Use In Diagnostic Procedures.
Ensure the success of your research withCST
IHC, ChIP, IF and WB application guides available to assist you with your research.
Download the guides to....
- Improve your results
- Select better suited antibodies
- Get easy-to-use protocols
- Use our recommendation to find the best companion products
© 2018 Cell Signaling Technology, Inc., 3 Trask Lane, Danvers, MA, 01923, United States. All Rights Reserved.
CEDARVILLE & Rockland Immunochemicals, Inc. Collaborate Together on a 3D Bioprinting
CEDARVILLE Engineering Group, LLC, (CEDARVILLE) and Rockland Immunochemicals, Inc., today announced a research collaboration designed to explore the development of innovative applications in 3D bioprinting that will advance life science research and accelerate the drug discovery process. Interest in 3D bioprinting has emerged in recent years. Applications for 3D bioprinting include drug screening, precision and regenerative medicine, food products and biosensors. The project is expected to leverage the expertise CEDARVILLE has in 3D modeling and printing, and the insight Rockland has in developing cutting-edge reagents and cell culture processes critical to novel biotechnologies.
“Every day, we help determine the planning and development of roadways, bridges, utility expansion and industrial or commercial sites. In that macro context, we have developed processes to create 3D scalable models with precision and accuracy to fit the workflows in that paradigm.” says April M. Barkasi, PE, President and CEO of CEDARVILLE. “This project presents an exciting twist. We will envision, now in a microenvironment, the application of technology and protocols on a cellular level to help advance scientific discovery in medicine.”
“The 3D bioprinting market is expected to grow in excess of 25% over the next five years to reach $1.33B in 2021.” says Dr. Karin Abarca, Vice President, Science Operations, at Rockland. “Our extensive know-how in 3D cell culture and the production of bespoke reagents critical to drug discovery provide a keen perspective on the opportunities and promise of 3D bioprinting. Rockland partners with industry leaders to target attractive markets with critical unmet needs. Together with CEDARVILLE, we foresee that our multi-disciplinary team will make real and meaningful advances in 3D bioprinting.”
Immunostar offer 10% discount all primary antibodies
We are excited to offer a 10% discount on all primary antibodies now through Feb. 28, 2018.
Use Coupon Code “ARN2018-10%” to receive up to ten antibodies for 10% off.
You may order a variety of antibodies or the same antibody. For online orders type “ARN2018-10%” in the comment section. Adjustments will be applied and reflected in your confirmation invoice. (The cart does not automatically apply coupons.)
This offer is not available with other discounts. Distributors are excluded from this offer. Limit 10 vials per customer.
Microbiome Therapeutics Conference
Bio-Rad Webinar: Apoptosis "A question of Life or Death”
Apoptosis, or programmed cell death, is an essential process that ensures an organism’s health by eliminating damaged or aberrant cells.
The process is induced by a variety of stimuli and mediated either through cell surface death receptors (extrinsic pathway) or the mitochondria (intrinsic pathway) (Galluzzi et al. 2008).
Our webinar entitled “A question of Life or Death” provides an overview of the different modes of apoptosis and what antibody applications and assays to use to differentiate between healthy and apoptotic cells.
Special focus will be placed on explaining the different stages of apoptosis and what experimental read-outs to use to determine if a cell has passed “the point of no return”.
2nd Annual Next Gen Immuno-Oncology Congress

2ndAnnual Next Gen Immuno-Oncology Congressto be held on13th and 14th March 2018inLondon, UK.The congress aims tobring academicians, researchers and scientists from researchinstitutespharmaceutical,bio-pharmaceuticaland biotechnology companies to discuss the latest updates in the development of ADC’s, Bispecific Antibodies and Immune Checkpoint Inhibitors.
Antibody Resource Partners with Enago to Offer Manuscript Preparation Services
24 October 2017, NEW YORK: Antibody Resource announced their partnership with Enago, a leading author service provider that specifically caters to the large section of the global research community for whom English is a second language (ESL).
Antibody Resource is the premier source of antibody and ELISA (enzyme-linked immunosorbent assay) products from major suppliers. Every day thousands of researchers from all over the world use the service to obtain the most up-to-date information on products and services available for antibody and ELISA related research and practice.
Enago is a trusted name in author services for the global research community. Since 2005, Enago has worked with more than 100,000 researchers in 125 different countries, improving the communication of their research and helping them to achieve success in international publication. Enago is the preferred partner for leading academic publishers, societies and universities worldwide. Enago has offices in Tokyo, Seoul, Beijing, Shanghai, Istanbul and New York, and operates globally with regional teams supporting researchers locally.
“Through this partnership we can provide an important benefit for our users all over the world who may at times struggle with the process of creating English language content, such as submitting their research to a scientific / medical journal. By offering Enago’s language editing services, we hope we can help any Antibody Resource user when writing important documents in English, in particular those for whom English isn’t their first language. ” commented Jonathan Baron, Commercial Director, Antibody Resource.
“Enago is delighted to collaborate with Antibody Resource to help serve the needs of the global antibody community by providing easy access to high quality manuscript preparation services. We are pleased to support Antibody Resource user ESL authors by helping them to overcome language barriers and enrich their content,” said Rajiv Shirke, Vice President for Global Operations at Enago.
For further information, visit the website: https://www.antibodyresource.com/
About Enago
Enago is the trusted name in author services for the global research community. Since 2005, we have worked with researchers in more than 125 countries improving the communication of their research and helping them to achieve success in publication. Enago is a preferred partner for leading publishers, societies, as well as universities worldwide. We have offices in Tokyo, Seoul, Beijing, Shanghai, Istanbul, and New York. Enago operates globally with regional teams supporting researchers locally.
To learn more about Enago, please visit: enago.com
About Antibody Resource
Antibody Resource is the premier source of antibody and ELISA products from the major suppliers located near Cambridge UK. It provides information on 2 million antibody products from 170 suppliers covering 27,000 proteins. Antibody Resource is spearheading a unique initiative designed to compare antibodies from numerous suppliers using identical samples/tissues and an identical protocol. In doing so, it can enable scientists to form an unrivalled opinion of which is the most suitable antibody for their research and in particular, which is going to require the least amount of optimisation, a process which can often take weeks or months.
For more information, please contact:
Robert Smith
Email id: robert.smith@enago.com
Phone number: +1 (540) 449 2067
1732, 1st Ave #22627
New York, 10128
Website: www.enago.com
BBI Solutions adds more antibodies to product range

BBI Solutions has continued to invest in its portfolio of antibodies and today announced the launch of 10 infectious disease markers, 3 inflammatory markers, 2 fertility markers and an additional cardiac marker. All the antibodies have been tested in either lateral flow or ELISA and are available for sampling today.
BBI Solutions (BBI) is in a rapid period of expansion and growth with recent announcements of a £14m investment into a global headquarters for the BBI Group and a recent acquisition of Maine Biotechnology Services. This further investment in to the expansion of BBI’s antibodies portfolio forms another part of the company’s growth plans as they continually aim to meet the growing demands of their customers.
BBI’s new infectious disease markers includeH. pylori, Influenza A and B,E. coliO157,CampylobacterandLegionella pneumophila. BBI have also released antibodies for three inflammatory markers; Calprotectin, CRP and IL-6, two fertility markers; AMH and Estriol and Myoglobin antibodies as a cardiac marker.
James Steggles, BBI’s Antibodies Commercial Manager explained “we are pleased to be able to offer this range of high quality antibodies that test developers can use with confidence as part of their research and development assay programs. As they aim to improve diagnosis of key diseases and conditions.”
BBI’s mission is to deliver exceptional products and technologies that people rely on to enjoy a better quality of life. “We have selected these antibodies following requests from our customers. They also work towards achieving our mission and our growth plans”, continued James.
All of BBI’s new antibodies have either been tested in lateral flow or ELISA assays, many are already being used in commercial assays and there are a number of recommended matched pairs, which will save time and money in customer’s screening processes. To discover more about these antibodies and their matched pairs or to order a sample visit our website or call today +44 (0) 2920 747 232
3 for 2 on Primary Antibodies from Abnova
3 for 2 offer is running to the end of September. Click here to find out more.
Chance to win 10 Free Sample Size Antibodies from Novus Biologicals
There are over 17,000 Sample Size Antibodies to choose from, click here for more details
Apoptosis Detection kit - Early and Late Cell Death
Apoptosis is a regulated process of cell death that occurs during embryonic development as well as maintenance of tissue homoeostasis. The appearance of non-regulated apoptosis involves the possibility of different diseases, such as neurodegenerative disease and cancer.
Abbexa supplies a range of apoptosis kits available in a variety of Fluorochromes:
What are the benefits?
Our assays are highly sensitive and can detect early to late events, offered in a range of different fluorochromes. Validated in flow cytometry with many different cell types including, Jurkat, HL60, U266, whole blood and astrocytes.
Validated with a range of drugs including Cytarabine, Camptothecin, Etoposide, Methotrexate.
Different fluorescent options for multiple laser excitation sources
abx290007
Apoptosis Detection Kit with PI (FITC)
abx290009
Apoptosis Detection Kit (PE)
abx290010
Apoptosis Detection Kit (Dylight 634)
abx290012
Apoptosis Detection Kit (CF-Blue)
Protocol

Our information page on apoptosis has more details about the process and products available.
Want to uncover mechanisms regulating autophagy in your system
Learn more about validated antibodies for epigenetic research.
Peptides for the Potent and Specific Induction of Autophagy
Do you know about specific ways to induce autophagy?
New Flow Cytometry Antibodies
Flow Cytometry New Antibodies

United States Biological is excited to announce new Flow Cytometry antibodies!
Flow cytometry is a widely used method for analyzing the expression of cell surface and intracellular molecules, characterizing and defining different cell types in a heterogeneous cell population, assessing the purity of isolated subpopulations and analyzing cell size and volume. It allows simultaneous multi-parameter analysis of single cells.
For more about this promotion, ClickHere.
CRISPR/Cas9 Superstar Kit
Given all the interest and buzz around CRISPR, please check out our panel of CRISPR antibodies from market leading suppliers.
You can buy a collection of sample sizes from different suppliers and test them before you go on to buy the full size vial.
If you have any questions, please send us an e-mail at antibody@antibodyresource.com
How to make a scientific poster
Making a scientific poster for an upcoming conference can be incredibly daunting. Where do you start? What do you include? How should you organize it? No need to fret about it. We’ve got you covered!
I know many people have differing opinions on the best program to use to make a poster, but I like simplicity, and that’s why I’ve always used PowerPoint for all of my scientific posters.
First determine the size you need your poster to be.Each conference should specify the maximum poster size allowed. Then set the page size of your document to the actual full size that you want the poster to be printed at.
This should be the first thing you do, as changing your page size after adding content will result in hours of additional formatting.
Want to just fill in your information without worrying about formating?Have a look at this great powerpoint scientific poster template at the standard size of 48″x36″. It comes complete with content ideas for each section, as well as some powerpoint tips and tricks.
Click below to download the template:
Now let’s get down to the nitty-gritty of making a scientific poster!
1. Scientific Poster Guidelines –Include these key sections:
a. Title –Write it as a sentence. Do not capitalize the first letter of each word.
b. Introduction–Be brief and only include essential information.
c. Materials and Methods –Break it into subsections with images or diagrams. Or use a flow-chart.
d. Results –Break it into subsections with headers summarizing the key findings for each set of graphs.
e. Discussion/Summary–Make a diagram outlining the major differences you found.
f. Conclusions/Future Directions –A few brief bullet points will bring home your message.
g. Literature cited –Only include a few key citations from your introduction or methods.
h. Acknowledgements –Thank the people that deserve thanking. Include funding agency logos here.
2. Create a title that can be easily read from a few feet away.Use a sans-serif font (Arial, Calibri) in bold. These are easier to read for large titles.
3. Use columns to organize your information, not rows.Depending on the size of your final poster, you may want 3-4 columns.
4. Use large headers to outline sections and break-up text.I’m fond of coloured blocks with white writing. These also help delineate the columns without using a border for each column (which is a bit dated).
5. Use bullet points.Be brief with your text. Less is more. Bullets should not run more than 2-3 lines.
6. Do not use a busy background.Stick with a simple white or solid colour background. You do not want to distract from your data.
7. Use black or dark font.Stay away from coloured text as it is difficult to read.
8. Add lots of images.If you are getting into more than 4-5 lines of solid text, stop and think about how you could summarize this better with a diagram or image.
9. Your graphs should be simple,with axis titles that are legible from a reasonable distance (ie. 2-3 feet). Remove any background or gridlines as they obscure the data.
10. Export your images as png files and at the desired resolution.Do not resize them drastically inside your poster making program.
11. View your poster at fullsize on your screen,carefully checking out all images and text regions to make sure they are not pixelated.
12. Have a quick summary readyfor when people ask “so what did you do here?”
The most important thing to keep in mind is that people viewing your poster are just walking by, and you need to catch their attention with the title or imagery to get them to stop.
The poster design needs to be attractive, uncluttered and easy to read for this to work.
Then you only have a few seconds to convey important details before they move on. This is best done with graphs, diagrams or images. A good figure can convey paragraphs worth of information.
Now impress your PI by getting a head start on your poster for your next conference.
What conference are you attending next?
IgE turns 50
Find out about the history of IgE here
Request your ICC guide!
5th European Congress of Immunology
The 5thEuropean Congress of Immunology will take place in Amsterdam, the Netherlands fromSeptember 2-5, 2018. The theme of the meeting is Building Bridges. The biggest European immunology meeting attracts more than 3500 participants, but we will ensure that you can meet colleagues of your specific research fields by locating specific topics in dedicated and constant areas of the meeting.
On behalf of the various national immunology societies in Europe and EFIS, we invite you to discuss the latest developments in immunological research and in clinical immune modulatory therapies.
We will specifically dedicate sessions to identify new discoveries in immunology that hold diagnostic and therapeutic promise and sessions that tackle challenges arising when connecting fundamental and translational science. In addition, we will connect immunology to scientists from interdisciplinary fields, like data management and systems biology, multidimensional imaging, metabolic regulation and microbiology to form beachheads for novel directions of immunological research and optimised evaluations of clinical trials.
Please, save the date. We are looking forward to welcome you to Amsterdam!
Talkin’ Immunology Podcast
Need to keep your lab humming and learn about science simultaneously? Now you can listen to BioLegend’s podcast to laugh and learn about new scientific discoveries and immunological innovations.
Check out the podcast here.
Internship Program at Rockland Immunochemicals Inc
Rockland Immunochemicals, Inc. offers an internship program for exceptional university students pursuing a career in biotechnology and related fields. Rockland seeks to encourage exemplary scientists early on and to provide opportunities in an industrial laboratory environment, engaging interns in hands-on, project-based training.
The interns are assigned by the internship coordinator to a mentor and a specific department, based on the applicant’s skills and knowledge. All interns will learn project and time management and will work with a team. The individual may assist in product manufacturing, development and implementation of biological manufacturing processes, scale-up procedures and testing methods relevant to the production of monoclonal antibodies and other biologics.
Unique Humanised Antibodies for Zika Diagnostics
Unique Humanised Antibodies for Zika Diagnostics

Assay developers regularly use patient sera as positive controls in their assays. These are excellent reagents where the sera employed are characterized and do not contain cross-reactive antibodies to other infectious diseases that need to be excluded from a differential diagnosis.
Challenges
One of the major challenges facing assay developers in their quest to prepare high quality immunoassays for detecting infection with Zika virus is the lack of suitable patient controls that contain IgG or IgM antibodies recognizing Zika virus antigens.
Our Solution
In order to overcome this challenge, United States Biological have utilized their leading monoclonal antibodies specific to Zika NS1 to prepare humanized versions with human IgG and IgM heavy chains. These unique reagents may be used to create defined controls in assays detecting Zika infection by measuring the human IgG and IgM response.
For more about this promotion, Click Here.
Atlas Antibodies Appoints John Daicic as New CEO
Atlas Antibodies, based in Stockholm, Sweden, today announces that John Daicic has been appointed new Chief Executive Officer effective August 7, replacing Marianne Hansson who will leave the company.
John Daicic joins Atlas Antibodies from his role as Acting General Manager for BioProcess Downstream Hardware at GE Healthcare in Uppsala, Sweden. John holds a PhD in Physics from the University of Melbourne in Australia and is Associate Professor in Surface Chemistry at the Royal Institute of Technology (KTH) in Stockholm. John has extensive experience from various leading roles within research and development as well as business development in the Life Science field.
“We are extremely pleased to be able to welcome John as the new CEO of Atlas Antibodies” says Mathias Uhlén, chairman of the Board of Directors and continues: “John’s background in research and long experience in marketing and business development makes him the ideal person to lead the company to the next stage of growth.”
Atlas Antibodies have since its foundation in 2006 made a journey from a start-up company to an international player with 98% of the sales in export from more than 60,000 products aimed for researchers in industry and academia.
Atlas Antibodies is well recognized as number 1 in IHC, providing reproducible polyclonal antibodies to more than 10,000 customers in 60 countries. Atlas Antibodies has a great reputation in the field of research antibodies and large potential to continue increasing the market share. New products such as the QPrEST product line for protein quantification will increase the revenue growth even further.
Marianne Hansson, who now is stepping down as CEO, has been leading the company from the start and during her period the company has been rewarded multiple times for entrepreneurial achievements, including three times on the 33 list of technology companies awarded by Ny Teknik and three times Gasell winner awarded by Dagens Industri.
“On behalf of the Board of Directors, I thank Marianne Hansson for her remarkable results in setting up Atlas Antibodies, growing the business and the excellent value she has created as CEO”, says Mathias Uhlén.
"Atlas Antibodies has made outstanding progress in commercializing the tremendous output of the Human Protein Atlas program, giving the benefit of a unique offering of antibody reagents to research and industry customers globally," says John Daicic. "I'm excited to join the Atlas Antibodies team and am looking forward to working together to create further value for customers and stakeholders as the company goes into the next important stage of its development."
About Atlas Antibodies:
Atlas Antibodies started 2006 as a start-up from the Human Protein Atlas project, and has since the start successfully launched more than 60,000 products for protein research. The company was founded by researchers at the Royal Institute of Technology (KTH) in Stockholm and the Rudbeck Laboratory, Uppsala University in Uppsala to handle the production, marketing and sales of research tools developed by the Swedish-based Human Protein Atlas program. Today the company offers four product lines: Triple A Polyclonals, PrecisA Monoclonals, PrEST Antigens and QPrESTs. QPrESTs are mass spectrometry standards for absolute quantification of proteins.
Introduction to Epigenetics
Introduction to Epigenetics
 Research in epigenetics helps to unravel the mysteries of major biological processes such as cancer, aging, and the development of diseases. It helps us understand how the environment might impact our gene expression and advances the traditionally-held view of the "code of life." Best of all, researchers can start out with little more than DNA, histone proteins, or chromatin and investigate fascinating topics such as cancer epigenetics, neuropsychiatric disorders, nutritional development, metabolic diseases, novel therapeutics, and more - no matter what discipline they specialize in.
Research in epigenetics helps to unravel the mysteries of major biological processes such as cancer, aging, and the development of diseases. It helps us understand how the environment might impact our gene expression and advances the traditionally-held view of the "code of life." Best of all, researchers can start out with little more than DNA, histone proteins, or chromatin and investigate fascinating topics such as cancer epigenetics, neuropsychiatric disorders, nutritional development, metabolic diseases, novel therapeutics, and more - no matter what discipline they specialize in.
The progress of epigenetic product development over the years has enabled low-cost exploration of epigenetic modifications, so it's easier than ever to gain a significant insight into samples a researcher may already be working with in the lab. Checking for epigenetic modifications could lead to the publication of new insights that might not have been previously considered.
 Epigenetics-related publicationsin PubMed have risen exponentially and continue to grow. Green line: Epigenetic articles. [Data collected from PubMed]
Epigenetics-related publicationsin PubMed have risen exponentially and continue to grow. Green line: Epigenetic articles. [Data collected from PubMed]
What is Epigenetics?
Epigeneticsis the covalent modification to DNA that impacts gene expression without affecting the underlying genetic sequence. These various epigenetic marks act "above" or "on top of" the DNA code to influence the expression of genes. With advancements in next-generation sequencing (NGS), researchers are able to delve deeper into the nuances of their samples and determine where modifications lie at base-resolution.
» Learn more about epigenetics and keep up-to-date on interesting new research at:www.whatisepigenetics.com
It's well known that this emerging field is becoming the focus of many researchers and labs across the world. Epigenetic-related publications have been rising exponentially since the 2000s. Not only is the research space predicted to expand, but a larger number of grants for epigenetic research will likely be awarded. A significant portion of research in epigenetics focuses onDNA methylation(as well as its subsetDNA demethylation), the most well-characterized epigenetic mechanism, and this is an ideal place to start for those just beginning. Scientists can make their mark in epigenetic research by investigating this and many other epigenetic modifications.
Do you have genomic DNA, chromatin, or histone protein samples? Then you're ready to get started in epigenetics!
» See a complete offering ofsample preparation kitsfor DNA, protein, and chromatin prep using a variety of starting materials and input amounts.
DNA Methylation
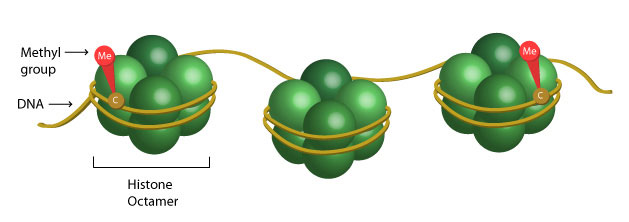
DNA Methylationoccurs by the addition of a methyl group (CH3) to DNA.
DNA methylation is defined as the addition of a methyl group to DNA, specifically cytosine, by an enzyme known as DNA methyltransferase (DNMT), thereby affecting gene expression. This epigenetic mark typically reduces the expression of genes and is involved in normal processes such as aging and cell differentiation, but is also thought to play a role in the development of diseases such as cancer, Alzheimer's disease, and many others. As the first epigenetic mark to be discovered, DNA methylation continues to be a major focus of numerous epigenetic researchers.
Global DNA Methylation
Total 5-mC levels


Easily jump-start your epigenetic research by looking at global DNA methylation.
The easiest way to begin research in epigenetics is by investigating global DNA methylation. Measuring total 5-methylcytosine (5-mC) levels may reveal possible epigenetic factors at play and offer invaluable insight into your samples. Global DNA methylation can vary between sample sets such as diseased versus healthy, between various tissue or cell types, and even between identical twins. Global hypomethylation and hypermethylation are linked to cellular processes such as aging, tumorigenesis, and disease development.
It has been implicated as a biomarker in numerous human cancers and could help assess neurodegenerative diseases such as Alzheimer's and Parkinson's. Importantly, research has shown that decrease in global DNA methylation is one of the most important characteristics of cancer.
In addition, global DNA methylation quantification is an excellent pre-screening tool that many researchers carry out prior to time-consuming and often costly sequencing, which is typically the path one would pursue for more in-depth investigation.
 See aglobal DNA methylation methods comparison chartfor an overview of the advantages, disadvantages and capabilities of global DNA methylation methods, including HPLC, LC-MS/MS, LUMA, and ELISA-based assays.
See aglobal DNA methylation methods comparison chartfor an overview of the advantages, disadvantages and capabilities of global DNA methylation methods, including HPLC, LC-MS/MS, LUMA, and ELISA-based assays.
Commonglobal DNA methylation methodsinclude HPLC, LC-MS/MS, LUMA, and M.SssI acceptance assays. HPLC uses enzymatic hydrolysis of genomic DNA and typically requires large amounts of sample DNA. Furthermore, optimization of the procedure requires extensive expertise. LC-MS/MS is considered the "gold standard" for quantification of global DNA methylation and similarly digests and separates deoxyribonucleotides, but analysis by mass spectrometry requires highly specialized knowledge. LUMA is a method that relies on cleaving the DNA with methylation-sensitive restriction enzymes followed by pyrosequencing. It isn't conclusive as to whether or not the results from this method accurately represent global DNA methylation. Lastly, the M.Sssl acceptance assay requires radioactivity for analysis and utilizes CpG DNA methyltransferase M.SssI and methyl donor S-adenosylmethionine. Unfortunately, this assay often leads to large intra- and interassay variability if the enzyme activity or stability of the methyl donor SAM is sub-optimal.
Unfortunately, this assay often leads to large intra- and interassay variability if the enzyme activity or stability of the methyl donor SAM is sub-optimal.
Directly quantifying total 5-mC for an accurate measurement of global DNA methylation can be accomplished in under 4 hours using the MethylFlash technique, a simple ELISA-like method:
- MethylFlash Methylated (5-mC) DNA Quantification Kit
Quantify global DNA methylation by specifically measuring 5-mC levels
Recent Research:

DNA Methylation Modulates Nociceptive Sensitization after Incision.
Sun, Y et al. (2015). PLOS ONE.
In this global DNA methylation study, researchers investigated the role of DNA methylation in modulating pain after incision and identified methylation-regulated targets contributing to incisional pain including the Oprm1 gene and others. The researchers measured global DNA methylation using theMethylFlash Methylated DNA Quantification Kit.
»See more global DNA methylation studies.
A global DNA methylation assay is a fast and inexpensive pre-screening method for those just starting their epigenetic study.
Methylation-specific PCR (MSP)
Gene-specific, base resolution DNA methylation
 What You Need:
What You Need:
1)BisulFlash DNA Modification Kit
2)MethylAmp MS-qPCR Fast Kit
3) PCR Instrument
A slightly more advanced method for beginners looking to start in DNA methylation research is gene-specific, base-resolution DNA methylation analysis. This assesses the amount of DNA methylation of a specific gene at base resolution, compared to global DNA methylation, which provides an overall view of the entire methylation status of the genome. TheBisulFlash DNA Modification Kitis the ideal kit for quick bisulfite treatment of DNA geared towards methylation-specific PCR analysis. MSP begins with bisulfite conversion wherein unmethylated cytosine is converted to uracil. In subsequent PCR amplification, uracil is recognized as thymine, leaving the methylated cytosines (5-mC) unaffected. This allows the amount of methylation to be measured. Protecting against DNA degradation caused by the typically harsh chemical reaction is vital in order to ensure optimal recovery and accurate methylation status.
Use these methylation-specific PCR resources to enhance your epigenetic study:
- Methylation-Specific PCR
Ku, J. et al., Epigenetics Protocol, 2011 - Optimizing Methodologies for PCR-based DNA Methylation Analysis
Hernández, H.G. et al., Biotechniques, 2013 - DNA Methylation Analysis PCR Primer Database
Ghent University, Faculty of Medicine and Health Sciences, Centre for Medical Genetics
DNA Bisulfite Sequencing (Methyl-Seq)
Genome-wide, base resolution DNA methylation
 What You Need:
What You Need:
1)EpiNext High-Sensitivity Bisulfite-Seq Kit (Illumina)
2) Illumina MiSeq, MiniSeq, NextSeq, or HiSeq
For those just beginning epigenetic research, next-generation sequencing (NGS) may seem daunting. There is typically a lot of data produced and significant time must be devoted to interpretation and figure generation. Massively-parallel sequencing platforms, such as Illumina or PacBio, have become invaluable tools that revolutionized genome and epigenome analysis. NGS gives researchers a deeper and far more detailed look into their samples than ever before. NGS technology delivers robust, unprecedentedly high-quality data.
Common DNA methylation-based applications include whole genome (WGBS), reduced representation (RRBS), and targeted bisulfite-seq. Successful bisulfite sequencing projects start with complete and efficient bisulfite conversion. Samples can be sequenced on an Illumina instrument for precise identification of methylated genes of the sample. TheEpiNext High-Sensitivity Bisulfite-Seq Kit (Illumina)is ideal for streamlined bisulfite sequencing NGS library preparation from low input DNA. Because it’s an all-in-one kit, it includes everything needed for bisulfite conversion and library preparation and is excellent for beginners.
Histone Modifications
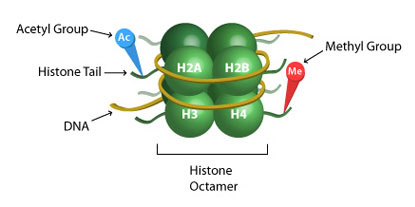
Histone modificationsoccur by adding an acetyl group (histone acetylation) or a methyl group (histone methylation) to the histone tails via specific enzymes.
Investigatinghistone modificationsis another route to pursue for those just getting started in epigenetic research. Post-translational modifications (PTMs) which occur to histones are thought to play a crucial role in transcription repression or activation. Such histone modifications include histone methylation, histone acetylation, and histone phosphorylation. These epigenetic mechanisms and the way they impact chromatin structure or recruit histone modifiers brings us closer to understanding the factors that alter gene expression and the consequences of these changes.
Histone modifications can impact the structure by loosening or tightening chromatin. Looser chromatin is known as euchromatin, which is more accessible to transcription and increases gene expression. Heterochromatin, on the other hand, is more tightly compacted, making it less accessible to transcription and, as a result, reducing gene expression. New insights into different histone modifications and their effect on cellular processes begin to unveil what some refer to as the “histone code.”
Global H3 or H4 Modification ELISA
Total quantification of well-characterized H3 or H4 modifications
 What You Need:
What You Need:
1)EpiQuik Histone H3 Modification Multiplex Assay KitorH4 Modification Multiplex Assay Kit
2) Illumina MiSeq, MiniSeq, NextSeq, or HiSeq
Similar to beginning in global DNA methylation research, when getting started in histone modifications it is very useful to have an overview of your samples. This will save time and effort in the future and can help narrow down areas for further experimentation.
Investigate 21 different histone H3 modifications or 10 different histone H4 modifications on one microplate using theEpiQuik Histone H3 Modification Multiplex Assay Kitor theEpiQuik Histone H4 Modification Multiplex Assay Kit. This high throughput method will accurately quantify amounts of identified histone proteins at specific H3 or H4 modification sites present in your sample prior to getting involved in more advanced downstream applications such as ChIP-sequencing (ChIP-seq).
If you have a single histone methylation or histone acetylation modification you’d like to measure, use thesehistone methylation quantificationandhistone acetylation quantificationkits. Popular kits include:
- EpiQuik Global Tri-Methyl Histone H3K27 Quantification Kit (Colorimetric)
- EpiQuik Global Acetyl Histone H3K9 Quantification Kit (Colorimetric)
- EpiQuik Global Histone H3K27 Methylation Assay Kit
- EpiQuik Global Histone H3 Acetylation Assay Kit
Chromatin Immunoprecipitation
Gene-specific identification of histone modifications
 What You Need:
What You Need:
1)ChromaFlash High-Sensitivity ChIP Kit
3)EpiQuik Quantitative PCR Fast Kit
4) PCR Instrument
Chromatin immunoprecipitation, or ChIP, is a popular epigenetic research method for investigating protein-DNA binding interactions. This method can be used for identifying histone modifications at the gene-specific level. Chromatin immunoprecipitation involves the binding of proteins to particular DNA sequences, allowing researchers to investigate transcription factors that interact with target genes and histone modifications occurring at genomic locations. Briefly, it involves crosslinking proteins to DNA, shearing the chromatin viasonication, and immunoprecipitating with a specific antibody. Then, the protein-DNA complexes are reverse crosslinked so the DNA fragments can be analyzed. Various downstream applications of ChIP include ChIP-sequencing, ChIP-PCR, and ChIP-on-chip (microarrays). The ChromaFlash High-Sensitivity ChIP Kit is optimal for immunoprecipitation of chromatin (ChIP) from small amounts of mammalian cells or tissues.
![]() For an in-depth technical guide to ChIP, seeA Starter Chromatin Immunoprecipitation (ChIP) Protocolat What is Epigenetics? or read the three-part seriesA Technical Guide to Conquering ChIPby David Esopi, research specialist at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center.
For an in-depth technical guide to ChIP, seeA Starter Chromatin Immunoprecipitation (ChIP) Protocolat What is Epigenetics? or read the three-part seriesA Technical Guide to Conquering ChIPby David Esopi, research specialist at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center.
ChIP Sequencing
Genome-wide mapping and identification of histone modifications.
 What You Need:
What You Need:
1)EpiNext ChIP-Seq High-Sensitivity Kit (Illumina)
2) Histone antibody of choice
3) Illumina MiSeq, MiniSeq, NextSeq, or HiSeq
This method combines chromatin immunoprecipitation (ChIP) with massively parallel sequencing technology to precisely analyze protein interactions with DNA. ChIP-Seq is used for pinpointing the exact gene location to which the protein of interest binds, but like bisulfite sequencing, produces significant amounts of data. Use theEpiNext ChIP-Seq High-Sensitivity Kit (Illumina) Kitto perform ChIP and library preparation in a single kit.
Epigenetic Services
Explore the epigenome the easiest way possible with our comprehensive epigenetic services, which can be completed from sample preparation to bioinformatics. Just send us your samples and we will send you publishable figures and results, along with personalized technical support at your fingertips.
Our epigenetic services include:
- Methyl-Seq
Bisulfite-based DNA methylation sequencing - ChIP-Seq
Genome-wide chromatin immunoprecipitation sequencing - Compound Screening
Enzyme inhibitor screening and histone modification profiling
Researcher Contributions and Explorer Program
At Atlas Antibodies, we're interested in working together with our customers. We often get contacted by customers interested in testing our antibodies in samples or applications we have not validated ourselves. For this purpose we offer theExplorer Program, where customers get a 50% discount or 50% refund when sharing the results with us.
We would now like to introduce a feature on our website we call Researcher Contributions.This is a new tab on our product pages, were we present Explorer Program submissions and other data submission from our customers and other collaboration partners.With Researcher Contributions, you can share results from using our antibodies with us and other scientists.
Knockout & Knockdown Antibody Validation
Testing antibody performance against genetically modified samples
Validating an antibody’s specificity is crucial to ensuring the absence of nonspecific binding and, therefore, the highest level of functionality. Testing antibody performance against genetically modified samples is one way to verify that an antibody recognizes a specific target. This can be done using a variety of methods, including mouse knockout models, dominant negative mutants, morpholinos, siRNA, and most recently, gene editing.
Thermo Fisher Scientific is committed to providing the best antibodies available. We are currently retesting the entire portfolio of Invitrogen™ antibodies to verify that they are specific to the indicated targets.
Montreal Neurological Institute and Thermo Fisher Scientific Collaborate
Montreal Neurological Institute and Thermo Fisher Scientific Collaborate to Accelerate Neurodegenerative Disease Research Industry and academia to share expertise in effort to develop improved methods to produce and characterize antibodies and reagents for neurological research
The Montreal Neurological Institute (MNI) at McGill University, a world-leading institution focused on brain research and advanced patient care, and Thermo Fisher Scientific, the world leader in serving science, today announce an agreement designed to accelerate the understanding of neurological disease by focusing on about 30 proteins associated with Parkinson’s disease, amyotrophic lateral sclerosis (ALS), hereditary spastic paraplegias, epileptic encephalopathies and ataxias, which are all devastating brain diseases. According to the World Health Organization, neurological disorders and their consequences are estimated to affect as many as a billion people worldwide. With an aging population and lack of effective treatments for many of these conditions, it is critical to develop new tools and assays to advance research into the mechanisms of these diseases. Antibodies are invaluable for studying the function and location of proteins.
As part of the study, Thermo Fisher will generate antibodies for proteins that investigators at the MNI and other scientists have associated with particular neurological diseases. MNI investigators will generate cell lines lacking the proteins as a mechanism to validate the antibodies, and will then use their expertise in these disease domains, coupled with the Thermo Fisher-generated antibodies, to advance the understanding of these disorders. The research will result in a set of highly validated reagents for scientists to study neurodegenerative diseases. Thermo Fisher will utilize its ABfinity™ antibody technology to generate rabbit recombinant antibodies. These recombinant antibodies are derived directly from B-cells captured from immunized animals and have the advantage of being robust and reproducible. Scientists from both institutions will work together to characterize the reagents. Thermo Fisher will confirm definitive protein binding by identifying that theantibody binds the intended target through mass spectrometry and by testing the antibodies by Western blot and immunofluorescence using the cell lines lacking the proteins generated at the MNI as a control.
“We are pleased to partner with Thermo Fisher Scientific, a company that has a strong and proven reputation for its manufacture of antibodies,” said Dr. Peter McPherson, James McGill Professor at the MNI. “We are also delighted that Thermo is supportive of our Open Science Initiative and its mandate to make reagents and tools available to the community.” “This important work combines the expertise of industry and academia to develop and characterize world-class, robust tools to study diseases,” said Matt Baker, Director R&D and Business Development, Antibodies and Immunoassays at Thermo Fisher Scientific.
“By working with MNI scientists, our antibodies will be screened in the most relevant models by experts who are leaders in this field, resulting in high-value reagents for the entire research community.”
About the Montreal Neurological Institute The Montreal Neurological Institute and Hospital – The Neuro – is a world-leading destination for brain research and advanced patient care. Since its founding in 1934 by renowned neurosurgeon Dr. Wilder Penfield, The Neuro has grown to be the largest specialized neuroscience research and clinical center in Canada, and one of the largest in the world. The seamless integration of research, patient care, and training of the world’s top minds make The Neuro uniquely positioned to have a significant impact on the understanding and treatment of nervous system disorders. In 2016, The Neuro became the first institute in the world to fully embrace the Open Science philosophy, creating the Tanenbaum Open Science Institute. The Montreal Neurological Institute is a McGill University research and teaching institute. The Montreal Neurological Hospital is part of the Neuroscience Mission of the McGill University Health Centre. For more information, please visit www.theneuro.ca
About Thermo Fisher Scientific Thermo Fisher Scientific Inc. is the world leader in serving science, with revenues of $17 billion and more than 50,000 employees in 50 countries. Our mission is to enable our customers to make the world healthier, cleaner and safer. We help our customers accelerate life sciences research, solve complex analytical challenges, improve patient diagnostics and increase laboratory productivity. Through our premier brands – Thermo Scientific, Applied Biosystems, Invitrogen, Fisher Scientific and Unity Lab Services – we offer an unmatched combination of innovative technologies, purchasing convenience and comprehensive support. For more information, please visit www.thermofisher.com.
Thermo Scientific new high content screening poster
Get more data from your assays




Measure cytoskeletal rearrangement using Thermo Scientific™ high-content analysis tools and Invitrogen™ fluorescent reagents, enabling you to easily get reliable quantitative data.
Whether you’re counting actin fibers or measuring cell morphology, we’ve got what you need for robustresults:
•
Reagents and protocols
•
Peer-reviewed references
•
High-content instrument platforms
•
Optimised software tools for analysis
Use our high-content screening (HCS) multiplexing poster to help build your assays and answer more complex biological questions (i.e., track your target protein, monitor cell viability, or measure new proteinsynthesis).
Webinar: Fluorescence and compensation in flow cytometry
Fluorophores accept light at a specific wavelength and re-emit it at a higher wavelength, giving each fluorophore an excitation and emission wavelength. Although there is a peak or maximal emission the range is often several hundred nanometers. This can cause problems where unwanted light enters several detectors giving false positives. Compensation is required to remove this unwanted fluorescence. Careful choice of fluorophores can reduce the amount of compensation needed.
This webinar is recommended to novices and researchers with limited experience of flow cytometry.
This webinar will cover:
- Basic fluorescence principles
- What is compensation and why you need to apply it
- Compensation controls
- How to avoid compensation in small panels
- Common rules to help build more complex panels
Webinar: Fluorescence and compensation in flow cytometry
Date:Wednesday, April 5, 2017
Time:08.00 PDT | 11.00 EDT | 16.00 BST | 17.00 CEST
Presented by:Dr Mike Blundell, Product Manager at Bio-RadLaboratories
Featuring Question and Answer session. #bioradwebinars
Speaker Biography:
 Mike Blundell graduated from the University of Edinburgh with a B.Sc. in Immunology. He then moved to University College London where he joined the group of Prof. Adrian Thrasher and obtained his Ph.D. His thesis research was focussed on the primary immunodeficiency Wiskott Aldrich Syndrome. He investigated novel activating mutations and developed lentiviral vectors for use in gene therapy treatments, some of which have now been used in clinical trials. Mike contributed to 50 peer reviewed papers and has now left academia to become a product manager for Flow Cytometry.
Mike Blundell graduated from the University of Edinburgh with a B.Sc. in Immunology. He then moved to University College London where he joined the group of Prof. Adrian Thrasher and obtained his Ph.D. His thesis research was focussed on the primary immunodeficiency Wiskott Aldrich Syndrome. He investigated novel activating mutations and developed lentiviral vectors for use in gene therapy treatments, some of which have now been used in clinical trials. Mike contributed to 50 peer reviewed papers and has now left academia to become a product manager for Flow Cytometry.
Over 4,000 New Triple A Polyclonals Released Today
In apress releasetoday, we announce the addition of over 4,000 newTriple A Polyclonalsto our antibody catalog, all presented with additional data on the Human Protein Atlas. For each Triple A Polyclonal the correspondingPrEST Antigenis also released and available as a product today. You can nowfind over21,000 primary antibodies in our catalog,targeting 15,000different proteins.
To celebrate, we're offering 20% discount on all our antibodies. The offer is valid until March 15 on purchases directly from Atlas Antibodies with the promotion codeNEW20.
In today's product release, we include antibodies against new targets, antibodies tested in extended tissue panels in IHC as well asICC-IF validated antibodies from the newly released Cell Atlas on the Human protein Atlas.”This release expands our catalog by 8,000 products and brings us even closer to our goal of covering the complete human proteome of 20,000 protein-coding genes with highly validated antibodies”, says Dr Marianne Hansson, CEO of Atlas Antibodies.
17,000 of the Triple A Polyclonalsare validated in IHC and are available on the Tissue Atlas on the Human Protein Atlas. The Tissue Atlaspresents a complete map of protein expression in all major organs and tissues in the human body. Antibodies with product data from a number of new tissues never displayed before are included in today's launch, such as eye including retina, lactating breast, full sections of adrenal gland and new sections of the brain. Here are a few examples:

Eye/Retina, IHCstaining of human retina showsstrong positivity in photoreceptor cells usingAnti-PDE6B Antibody (HPA054148).

Lactating Breast, IHC staining of human lactating breast shows moderate cytoplasmic positivity in glandular cells usingAnti-LALBA Antibody (HPA029855).

Pituitary gland, IHC staining of human pituitary gland showsstrong membranous and cytoplasmic positivity in anterior lobe usingAnti-PRL Antibody (HPA042416).

Adrenal gland, IHC staining of adrenal gland showscytoplasmic positivity in medullary cells usingAnti-DBH Antibody (HPA002130).

Sweat duct cells, IHCstaining of human skin showsstrong membranous and cytoplasmic positivity in sweat duct cells and secretory cells usingAnti-DCD Antibody (HPA063967).
12,000 of the Triple A Polyclonalsare validated in ICC-IF, and presented on the Cell Atlas on the Human Protein Atlas. Here you can find high resolution confocal microscopy images for all antibodies and annotated subcellular location for the protein. The Cell Atlas presents a mapping for each gene to 32 subcellular structures, from organelles such as nucleus, golgi and mitochondria to sub-structures such as nuclear bodies, cytokinetic bridges and rods and rings. Here are a few examples:

Cytosol, ICC-IF staining of human cell line U-251 MG shows localization to cytosol usingAnti-CCNB1 Antibody (HPA030741).
Rods and rings, ICC-IF staining of human cell line U-2 OS shows localization to rods & rings usingAnti-ISL2 Antibody (HPA075192).
Aviva Systems Biology Acquires GenWay Biotech
"This acquisition will facilitate growth and enhance capabilities for both entities.GenWay's 18 years of experience producing proteins, antibodies, and ELISA assays will nicely complement ASB's objective to become a leading provider of proteomic solutions," saidPeter Jiang, M.D., ASB's Chief Executive Officer. "We see opportunities to expand both ASB's and GenWay's existing businesses as a result from this acquisition."
"We expect a smooth transition for both companies and their respective customers," saidMatt Landry, ASB's Vice President of Marketing and Sales. Prior to joining ASB in 2010, Mr. Landry was the Senior Vice President of Marketing and Sales for GenWay Biotech.He played a critical role in leading GenWay's product and service divisions from 2004 to 2010.
GenWay Biotech maintains ISO13485 accreditation for the development, validation, and production of antibodies and proteins for use in FDA regulated or CE marked products.The company also offers clinical laboratory services in a CAP accredited, CLIA facility. The company's services and products are offered to customers in research, diagnostic, and government institutions. This transaction will uniquely position ASB as a leader in product development, production, and services to assist customers with their research and diagnostic needs.
BAX Comparison Report published
The BAX Comparison Report has just been published, (please find the full report in the attached PDF). This report is part of ourseries continuing theComparison Initiativeto give you as a researcher another data point in order for you to make an informed decision about finding the right antibody for your experiment.
See the complete range of BAX antibodies here
These reports also feature antibody companies, you may never have heard about and allows them to compete against the market leaders and showcase their results in standardized experimental conditions.
There are two companies that wewould highlight and certainly be worth your consideration, SICGENand US Biologicalif you looking for a BAX Antibody, I know they would be happy to hear from you and answer any questions you may have.
As always we would welcome yourfeedback and if you haveany questions that you have about the report, please e-mailantibody@antibodyresource.com
What do the Mona Lisa and IHC have in common?

Download Novus’s IHC handbookto learn how to generate accurate expression and localization data in tissue
>Gain better insight into the multiple variables that impact IHC results>Follow step-by-step protocols from tissue preparation to IHC staining
>Troubleshoot typical problems to perfect your IHC assay


What do the Mona Lisa and IHC have in common?
Bio-Techne has assembled a collage of thousands of IHC and immmunocytochemistry (ICC) images depicting the Mona Lisa.
Downloadorrequest a free copyof the poster.
Inflammation
Inflammation describes a complex series of biological processes which the body initiates in response to certain stimuli that are usually, but not always, harmful to the organism. Although acute inflammation is necessary to rid the body of harmful pathogens, chronic inflammation can be destructive to healthy tissue and can both be caused by, and lead to, various diseases. Learn more about this critical process with BioLegend’s new webpage.
BioLegend Flow Cytometry Conjugates
BioLegend offers an extensive selection of antibody conjugates for flow cytometry. Now you can view them all with these handy reference charts to visualize fluorophore conjugate availability for each antibody clone.
Epitope Tag Reagents
When incorporated into the protein of interest, epitope tags make protein detection and identification very robust and easy. Now, the number of epitope tags has increased considerably. BioLegend epitope tag antibodies, peptide tags, and affinity gels are extremely specific, highly characterized and generate clear, quantitative results.
StressMarq invites you to evaluate their antibodies
Test any antibody* for free before you buy! Just pay for shipping! It's that easy!
StressMarq supplies the highest value bioreagents in the stress-related field. The performance of each has been documented and is supported with online datasheets. However, we cannot test each antibody in all applications or species. If our datasheets do not indicate whether our antibody is suitable for your application or species, we invite you to use our Trial Program! Shipping costs vary depending on your destination (approx. $30-80) or supply your own FedEx account number.
*Some exclusions apply. Excludes conjugated and lyophilized antibodies. Please inquire for sample size availability.*
Flow Cytometry Controls Guide
In flow cytometry, having the right controls is every bit as important as staining your samples. From compensation beads to autofluorescence and Fc blocking, our new webpage guides you multiple controls and the situations they're appropriate for. Learn what BioLegend offers to make sure your results are real and not artifacts.
Commercialization/Collaboration Program
At StressMarq Biosciences we constantly collaborate with leading researchers to bring new life science productsto the greater scientific community. If you have developed a new reagent that you would like to make available commercially, please contact our team atinfo@stressmarq.comto inquire about our commercialization and collaboration program.
Once we receive your email, we will work with you to determine the commercial viability of the reagent and develop a licensing agreement that is beneficial to both parties.
We are interested in licensing products that fit within our existing product range (antibodies, antibody conjugates, proteins, assay kits and small molecules), as well as new potential product types.
NEW PRODUCT SUGGESTIONS
Have you been looking everywhere for a particular product and are still unable to find it? Send us your suggestion for a new product, and we will put the team to work to see if it is viable.
You can be as involved as you would like to be, either just sending us your suggestion, or collaborating with us to develop and test the new reagent.Send usyour idea today!
WHY COLLABORATE WITH STRESSMARQ?
- Bring in money for your research:If your product is successfully licensed by StressMarq, you will receive potential license fees or royalty payments.
- Expand the reach of your reagents:Our global distribution network enables scientists around the world to easily purchase and conduct research using your reagent.
- Simple, profitable agreements:We provide flexible, straightforward licensing agreements that leave both parties happy!
Rockland Immunochemicals, Inc. Partners with Alex’s Lemonade Stand Foundation
In a continuing effort in the fight against childhood cancer, Rockland awarded the Joy Cappel Young Investigator Award (JCYIA) to two Alex’s Lemonade Stand Foundation (ALSF) Young Investigator grantees. In addition, Rockland is donating over $150,000 in reagents for the projects of ALSF Young Investigator and ‘A’ Award grantees with active grants.
The JCYIA includes the development of customized polyclonal antibodies by Rockland to further the research of Abhinav Dey, PhD (Young Investigator 2015) and Asen Bagashev, PhD (Young Investigator 2016). Dey and Bagashev received the award at the ALSF Young Investigator Summit in Chicago in October. Dey is researching personalized treatments for pediatric brain tumors. Bagashev is researching targeted therapies for relapsed B cell acute lymphoblastic leukemia.
The JCYIA, named in honor of former Rockland president and CEO Joy Cappel, is intended to foster research conducted by a promising post-doctoral fellow, graduate student or young investigator performing research in the areas of oncology, nuclear signaling, developmental biology, epigenetics, immunology, microbiology, neuroscience, signal transduction or stem cell technology. This award is in the form of a free-of-charge custom polyclonal antibody development project from Rockland.
Researchers investigating novel targets face a difficult hurdle when antibodies to their target are not commercially available. The JCYIA program gives researchers the opportunity to design a unique polyclonal antibody specific to their research, often resulting in a successful journal publication. Additionally, Rockland makes the antibody available to other researchers after validation as part of Rockland’s catalog antibody collection.
Since inception, ALSF has worked diligently to attract young doctors to the field of pediatric oncology, recognizing that these researchers hold future keys to better treatments and ultimately cures to end childhood cancers. The Young Investigator and ‘A’ Award grants reflect this belief. In addition to the two, ALSF funds several other grant categories to researchers on the front lines of the childhood cancer fight. For more information, visit: www.ALSFgrants.org.
About Childhood Cancer Childhood cancer is a general term used to describe cancer in children occurring regularly, randomly and sparing no ethnic group, socioeconomic class, or geographic region. Childhood cancer extends to over a dozen types of cancers and a countless amount of subtypes. Just a few of these cancer types include: Ewing’s sarcoma, glioma, leukemia, lymphoma, medulloblastoma, neuroblastoma, osteosarcoma, retinoblastoma, rhabdomyosarcoma and Wilms’ tumor. In the United States, childhood cancer is the leading cause of death by disease in children under the age of 15. Every day, approximately 250 kids around the world die from cancer, accounting for 91,250 losing their lives to the disease every year.
About Alex’s Lemonade Stand Foundation Alex's Lemonade Stand Foundation (ALSF) emerged from the front yard lemonade stand of cancer patient Alexandra “Alex” Scott (1996-2004). In 2000, 4-year-old Alex announced that she wanted to hold a lemonade stand to raise money to help find a cure for all children with cancer. Since Alex held that first stand, the Foundation bearing her name has evolved into a national fundraising movement, complete with thousands of supporters across the country carrying on her legacy of hope. To date, Alex’s Lemonade Stand Foundation, a registered 501(c)3 charity, has raised more than $127 million toward fulfilling Alex’s dream of finding a cure, funding over 650 pediatric cancer research projects nationally.
For more information on Alex’s Lemonade Stand Foundation, visit AlexsLemonade.org.
About Rockland Immunochemicals, Inc. Rockland Immunochemicals, Inc. supports the academic, biopharma, and diagnostic industries with antibodies and antibody based tools used in basic research, assay development, and preclinical studies. With facilities in Pennsylvania for over 50 years, Rockland manufactures products ideally suited for integration into critical assays such as western blotting, immunohistochemistry (IHC), immunofluorescence microscopy (IF), ELISA and flow cytometry. Additional information about Rockland’s life science tools and services can be found at www.rockland-inc.com
Phosphorylation and Phospho Proteins
Phosphorylation and de-phosphorylation play critical roles as a mode of signal transfer in biological processes. Check out our phosphorylation webpage to learn about this process, the proteins being phosphorylated, and the products offered at BioLegend to help you investigate this phenomenon.
Why Use Chicken IgY
There are several distinct advantages for using chickens over rabbits to produce your polyclonal antibodies.
1. Higher titres against highly-conserved mammalian gene products. Since chickens are at least 100 million years removed from mammals, they tend to recognize any mammalian gene product as foreign, and mount vigorous immune responses.
2. Double immunostaining is easier to perform. Chicken IgY’s can be used together with mouse and rabbit antibodies, without the danger of cross-reactivity. Secondary antibodies against chicken IgY’s don’t cross react with mammalian IgG’s.
3. Animal-friendly — we purify the antibodies from eggs, not serum. With rabbits, in contrast, serum is obtained by restraining the animals, and performing ear bleeds or cardiac puncture. It is simply a more humane way to produce antibodies.
4. Cheaper than the custom rabbit antibodies. Our chicken antibody preparations are >90% IgY, and have shelf-lives of 5 years or more at 4˚C. In contrast, rabbit serum (which is what most companies provide) has only a limited shelf-life at 4˚C (measured in weeks-to-months), and some biological activity is lost when frozen. The cost of having rabbit antibodies purified to comparable purities (i.e., protein A-purified) is very costly.
5. Nearly unlimited quantities (again, because the antibodies come from eggs). We collect about 18 “immune eggs” from each hen, but only use 6 “immune eggs” to prepare your IgY preparation. We then store the remaining 12 “immune eggs” in the refrigerator, in case you need more antibody down the road. Moreover, we can continue to house the hens (for an additional charge), in case you want us to perform additional injections.
In addition:
6. There is no “Fc domain” within the stem portion of a chicken IgY. This provides several advantages over rabbit IgG’s:
- It reduces the likelihood of having false positives in diagnostic applications, since it is the mammalian Fc domain that binds “rheumatoid factors” or other naturally occurring anti-Fc antibodies.
- Does not activate mammalian complement systems, allowing the use of chicken IgY’s in in vivo applications.
- Does not bind to mammalian Fc receptors, avoiding non-specific binding to cells expressing such receptors (e.g., macrophages and dendritic cells).
7. Chicken IgY’s contain a larger glycosylation index, allowing more labeling with HRP and other antibody tags. This produces higher signals.
Visit Aves Labs to find out more
Neuroinflammation Poster
BioLegend is proud to announce the release of our new Neuroinflammation Poster, which covers synapse pruning, demyelination, axon degeneration, brain blood barrier modification, aggregation, regeneration and more.
Learn more about neuroinflammation and view the poster at: www.biolegend.com/neuroinflammation
Kerafast Celebrates 5 Years of Facilitating Access to Research Reagents
Start-up company now partners with 140+ prominent research institutions
Boston, MA., December 13, 2016. Kerafast Inc., developers of an industry-leading online platform to facilitate accessibility to unique bioresearch materials from laboratories across the globe, is celebrating its five-year anniversary this December. Since its founding in December 2011, Kerafast has partnered with more than 140 prominent academic institutions to facilitate access to research tools created by their investigators. The company’s catalog now offers more than 2,600 lab-made research reagents, including antibodies, cell lines, probes and more, many of which are one-of-a-kind and unavailable elsewhere.
“Kerafast launched five years ago with a mission to advance life science research by promoting access to unique research reagents,” said Dr. Jennifer Rossi, Kerafast’s Chief Operating Officer. “We’ve since established a strong global community of researchers who are contributing, discovering and rapidly accessing sought-after reagents through the Kerafast online platform. We look forward to our continued growth and strive to support reagent transfer and scientific achievement for years to come.”
This past year, Kerafast continued to expand its international presence, with institutions in countries such as such as Australia, Canada, Germany, Israel and Norway joining the program to facilitate access to their research materials. In addition, Kerafast added several Zika virus-related reagents to its catalog in 2016, helping accelerate research into the global health threat by allowing scientists worldwide to easily obtain novel Zika materials for use in their own studies. Kerafast also expanded its fellowship program, supporting postdocs and graduate students in order to expose them to non-laboratory careers in the scientific industry. In another 2016 milestone, the company recently launched its new Kerafast.com website, which further streamlines the process of accessing unique lab-made reagents.
For five years, Kerafast has given back to science by returning generous royalty payments to the investigators and institutions that contribute their research tools. The company is constantly adding new reagents to its online platform, where the materials can be quickly purchased through a user-friendly research-use-only “click license.” To date, these reagents have been accessed and used by researchers in 53 different countries across six continents.
About Kerafast, Inc.
Kerafast has partnered with >140 prominent academic research institutions internationally that provide thousands of rare and unique ‘Reagents for the Greater Good’ for the benefit of the scientific community working toward the cure of disease in 53 countries on 6 continents. Visit kerafast.com for more information.
Antibody Drug Conjugates Market (3rd Edition), 2015-2025
INTRODUCTION
The ADC market is being driven by the sales of ADCETRIS® and KADCYLA®, the only two commercially available drugs. It is a relatively new concept with respect to technology, although the individual molecules that form ADCs have been used since long. This new class of therapeutics has witnessed a strong acceptance from both big and small pharmaceutical companies as they try to fill gaps in their respective oncology pipelines. There has been a healthy growth in the number of pipeline molecules (both clinical and preclinical), suggesting a heavy focus of stakeholders in the industry. Majority of the drugs are being developed for oncological indications. In fact, several molecules are designed to target a large bandwidth of cancers, focussing on multiple indications; such molecules form 19% of the clinical pipeline.
The field has been abuzz with partnering activities; such collaborations provide a synergistic boom to both the partners in terms of enrichment of product portfolio, revenues and stability. Technology licensing is the preferred model for ADC development. Seattle Genetics and ImmunoGen, the two leading technology developers, have been involved in a number of partnerships. In fact, asignificant proportion of ADCs in development are based on the ADC technology of these two companies.
The unexploited and promising nature of this market supports the hopes pinned on multiple start-ups by several strategic investors and venture capital firms. Over 80 instances of funding (equity + debt) were traced during our research, with the total investment amounting to an encouraging sum ofUSD 1.9 billionover the last decade. In addition to the venture capital firms, big pharma companies have also made strategic investment in smaller companies focused on developing ADCs.
A rich pipeline, promising safety / efficacy results and strong backing by venture capital firms is likely to result in a significant growth in the market over the next ten years. In the longer term, several start-ups / university spin-offs, which currently have molecules in preclinical / discovery phase, are expected to sustain the growth momentum.
SCOPE OF THE REPORT
The "Antibody Drug Conjugates Market (3rd edition), 2015-2025" report provides a comprehensive analysis of the current market landscape and future outlook of the growing pipeline of ADCs. The ADC market has been one of the most actively evolving markets over the last few years and encompasses a myriad of therapies that can be potentially exploited for a broad range of cancer indications.
One of the key objectives of the study is to review and quantify the opportunities laid by the robust, opportunistic and broad pipelines of the pharmaceutical firms. With two marketed drugs (ADCETRIS® and KADCYLA®), the market is treading its way towards fulfilling a huge untapped promise. Amongst other elements, the report elaborates on the following key areas:
- The current state of the market with respect to key players, developmental stage of pipeline products (both clinical / pre-clinical) and indications targeted
- Technology providers, and key cytotoxin, linker and conjugation technologies supporting the development of improved ADCs
- Recent partnerships that have taken place over the last decade including product co-development, licensing and clinical trial collaborations
- Various investments and grants received by the companies focused in this area
- Therapeutic areas forming the current focus of developers, the gradual drift and expansion towards broader application areas
- Competitive landscape and inherent threats to growth in the short and long term
- Development and sales potential, based on target consumer segments, likely adoption rate and expected pricing, of molecules in late stages of development
The base year for the report is 2015. The report provides market forecast for the period 2015-2025. The research, analysis and insights presented in this report include potential sales of several ADCs; this analysis is backed by a deep understanding of key drivers behind the growth.
Owing to niche nature of the market, with most products in the pipeline, we have provided three market forecast scenarios to add robustness to our model. The conservative, base and optimistic scenarios represent three different tracks of industry's evolution. All actual figures have been sourced and analysed from publicly available information. The figures mentioned in this report are in USD, unless otherwise specified.
RESEARCH METHODOLOGY
Most of the data presented in this report has been gathered via secondary research. For all our projects, we conduct interviews with experts in the area (academia, industry, medical practice and other associations) to solicit their opinions on emerging trends in the market. This is primarily useful for us to draw out our own opinion on how the market will evolve across different regions and technology segments. Where possible, the available data has been checked for accuracy from multiple sources of information.
The secondary sources of information include
- Annual reports
- Investor presentations
- SEC filings
- Industry databases
- News releases from company websites
- Government policy documents
- Industry analysts' views
While the focus has been on forecasting the market over the coming ten years, the report also provides our independent view on various technological and non-commercial trends emerging in the industry. This opinion is solely based on our knowledge, research and understanding of the relevant market gathered from various secondary and primary sources of information.
CHAPTER OUTLINES
Chapter 2 presents an executive summary of the report. It offers a high level view on where the ADC market is headed in the mid-long term.
Chapter 3 provides a general introduction to the ADCs. In this section, we have discussed, in detail, the concept of ADC, its components, mechanism of action and advantages over traditional therapies.
Chapter 4 provides a comprehensive landscape of the ADC market. This chapter has information on all the ADC molecules in development (clinical and preclinical) along with their current phase of development, key targeted indications, companies that are active in the field of ADC and most common types of cytotoxins and linkers used for ADC development.
Chapter 5 provides our analysis of the key opinion leaders (KOLs) focused in the ADC research sector. It provides a comprehensive list of principal investigators, along with their respective research institutes, and highlights KOLs who have relatively more experience.
Chapter 6 provides a competitive portfolio of various therapies being developed for the treatment of commonly targeted indications. These indications have been the prime focus of companies developing ADCs. The chapter also highlights the epidemiological facts and currently available treatment options for each indication.
Chapter 7 presents our analysis on the ADC market opportunity in the upcoming decade. It includes detailed drug profiles and likely sales forecast for drugs currently in phase II or higher stage of development. The profiles cover information such as drug's mechanism of action, history of development, clinical trial timeline, clinical trial results, manufacturing, estimated cost of treatment and dosage regimen.
Chapter 8 provides a comprehensive list of partnerships, including the details of agreements, which have taken place between various companies over the last few years.
Chapter 9 provides information on several funding instances that have driven research and development in the field of ADCs. Our analysis reveals interesting insights on the growing interest of venture capitalists and other stakeholders in this market.
Chapter 10 includes profiles of the key companies focused in the ADC market. Each company profile includes information such as financial performance, geographical presence, marketed / pipeline ADC drugs and recent collaborations.
Chapter 11 presents a case study on ADC manufacturing. The case study discusses the current manufacturing challenges associated with ADCs and provides information on the leading contract manufacturers that are focused in this domain.
Chapter 12 provides our analysis of the strengths, weaknesses, opportunities and threats in the ADC market, capturing the key elements likely to influence future growth.
Chapter 13 is a collection of interview transcripts; these discussions have helped us in forming a better understanding of the key market dynamics, competitive landscape and the likely future trends in the market. The companies interviewed include Piramal Healthcare,Pierre Fabre, PolyTherics, Oxford BioTherapeutics, Lonza, Catalent Pharma Solutions and BSP Pharmaceuticals. Two additional companies that were interviewed requested the details to be published as anonymous.
Chapter 14 summarises the overall report. In this chapter, we have provided a recap of the key takeaways and our independent opinion based on the research and analysis described in previous chapters.
Chapter 15 is an appendix, which provides tabulated data and numbers for all the figures provided in the report.
Chapter 16 is an appendix, which lists down all the companies and organisations involved in the ADC market.
EXAMPLE HIGHLIGHTS
1. Driven by the sales of ADCETRIS® and KADCYLA® (the only two drugs currently available commercially), the ADC market is estimated to be worth aroundUSD 0.9 billionin 2015.
2. The market has a strong clinical pipeline of 53 molecules; nearly one-third of the molecules are in Phase II or Phase III of development. The dynamic pipeline also includes a number of molecules in preclinical/ discovery stage; in our research, we came across over 60 such molecules.
3. Most commonly used cytotoxins for ADCs under clinical development include auristatin, calicheamicin, maytansine and duocarmycin. Auristatin dominates the market and accounts for over 50% of ADCs in clinical development.
4. Roche, with six molecules in clinical development, has the most developed pipeline of ADCs. Other established players in the market are (in alphabetical order) AbbVie, Amgen, Astellas, AstraZeneca, Bayer, BMS, GSK, Novartis, Pfizer and Sanofi.
5. Relative new entrants include (in alphabetical order) AbGenomics, Allozyne, Ambrx, Biotest, Celldex Therapeutics, Centrose, CytomX Therapeutics, Esperance Pharmaceuticals, Formation Biologics, Genmab, Heidelberg Pharma, Igenica, Immunomedics, Oxford BioTherapeutics, Kairos Therapeutics, Merrimack Pharmaceuticals, Mersana Therapeutics, NBE Therapeutics, Philochem, PhotoBiotics, Progenics Pharmaceuticals, Sorrento Therapeutics, Stem CentRx, Sutro Biopharma, Synthon, Zymeworks; emergence of these firms is likely to provide the necessary push, both in terms of technology and innovation.
6. About 70%-80% of ADC manufacturing is currently outsourced. There are limited number of contract manufacturers with capabilities for development of linkers and cytotoxins. In addition, even fewer CMOs provide conjugation services for ADC.
7. Several technological developments have taken place in the recent past; more stable linkers and potent cytotoxins are likely to ensure that the next generation ADCs have improved safety/efficacy profile.
8. With around 10 new ADC commercial launches over the coming decade, we believe the overall market will be worthUSD 10 billionannually by 2025.Favourable market environment and regulatory regimes could result in an even steeper growth.
Read the full report:http://www.reportlinker.com/p02280919-summary/view-report.html
The antibody that normalizes tumor vessels
An important parcel must be delivered to the correct place. It is so important that it can be a question of life or death. However, uneven streets and missing railings risk to bring it off the road and leave it undelivered. This is not the trailer of a drama, but what happens to anti-cancer drugs traveling towards the tumor area, but unable to reach it because of dysfunctional blood vessels. The Center for Vascular Research, within the Institute for Basic Science (IBS) discovered that their antisepsis antibody ABTAA (Ang2-Binding and Tie2-Activating Antibody) also reduces tumor volume and improves the delivery of anti-cancer drugs. Published inCancer Cell, this study demonstrates that ABTAA restores the structural and functional integrity of tumor blood vessels in three different tumor models: breast, lungs and brain.
Blood vessels inside and around an established tumor can be described as a chaotic and dysfunctional labyrinth. While the inner walls of healthy blood vessels are surrounded and supported by endothelial cells and other cells called pericytes; in the established tumor, the endothelial junctions are broken apart and pericytes are also detached. Blood flow into and from the tumor is severely retarded and tumor vessels lacking an intact vessel wall become leaky. This microenvironment causes limited drug delivery to the tumor and leads to inadequate oxygen supply (hypoxia) and even metastasis. IBS scientists found that the antibody ABTAA normalizes the tumor vessels and hence, change the whole tumor microenvironment. "We call it normalization of tumor vessels, because it resembles closely the wall architecture of healthy, normal vessels," explains PARK Jin-Sung, first author of the study. And continues: "Tumor can adapt to hypoxia and get more aggressive, so we tried to prevent this transition by normalizing tumor vessels. ABTAA changes the whole tumor environment, oxygenation status and level of lactate, so that the immune cells and drugs can reach the core regions of the tumor more easily. In this way, we create a favorable ground for tumor treatment."
In an attempt to generate antibodies targeting the protein Ang2, which is specifically expressed by endothelial cells in stressful conditions like in tumor, the team unexpectedly discovered that ABTAA has a peculiar way of working and a dual function. ABTAA indeed not only blocks Ang2, but it is also able to activate Tie2 at the same time. Tie2 is a receptor present on the cell membrane of endothelial cells. ABTAA causes Ang2 to cluster together and to strongly activate Tie2 receptors. "If we activate Tie2, we can efficiently normalize tumor vessels, enhance drug delivery and change the whole microenvironment," explains KOH Gou Young, Director of the Center for Vascular Research.
Several pharmaceutical companies are developing Ang2-blocking antibodies to cure cancer. However, even if these antibodies significantly inhibit tumor progression, they do not stop tumor hypoxia. Moreover, most of the anti-cancer drugs target the tumor at its early stage, when tumors are still hard to diagnose. ABTAA, instead, works with tumors that are already rooted: "When the tumor is established, hypoxia is the main driver of tumor progression. So, if we eliminate hypoxia, we make the tumor more mild, by reducing its progression and metastasis," comments Koh.
IBS researchers tested ABTAA in mice with three different types of tumors that show high levels of Ang2: glioma (a type a brain tumor), lung carcinoma and breast cancer. They also compared the effect of ABTAA with ABA, another antibody that blocks Ang2 but misses the Tie2 activating properties. In all three cases, ABTAA was superior to ABA in inducing tumor vessel normalization, which led to a better delivery of the anti-cancer drugs into the tumor core region.
Glioma is one of the so-called intractable disease, because of its poor prognosis and treatment. IBS scientists found that the glioma volume was reduced 39% by ABTAA and 17% by ABA. ABTAA profoundly reduced vascular leakage and edema formation in glioma through promoting vascular tightening. Moreover, when ABTAA was administered together with the chemotherapeutic drug temozolomide (TMZ), the tumor volume reduces further (76% by ABTAA+TMZ, 51% by ABA+TMZ, and 36% by TMZ).
In the Lewis Lung Carcinoma (LLC) tumor model, the research team administered ABTAA together with a chemotherapeutic drug called cisplatin (Cpt) and observed a greater suppression of tumor growth (52%) compared with the controls and increased overall survival. Moreover, ABTAA+Cpt led to a marked increase in necrotic area within tumors.
Finally, in a spontaneous breast cancer model, ABTAA delayed tumor growth and enhanced the anti-tumor effect of Cpt.
In the future, the team would like to further understand the underlying relationship between faulty blood vessels and diseases. "We would like to apply this antibody to an organ that is rich in blood vessels, that is the eye, and see if this antibody can be useful to treat eye diseases such as age-related macular degeneration and diabetic retinopathy," concludes Koh.
Exploring Kidney Proteins & Antibodies
Which proteins are expressed in kidney? We take a look at theHuman Protein Atlas's analysis of the kidneyspecific transcriptome. Here we present highlights from the findings, together with selected antibodies against proteinsspecificallyexpressed in kidney. All antibodies are presented on the Human Protein Atlas and available in our catalog asTriple A PolyclonalsandPrecisA Monoclonals.
65% of all human proteins are expressed in kidney
Two thirds of all human proteins are expressed inkidney. The number is based on the Human Protein Atlas analysis ofRNA-Seq data for approximately 20,000 human proteins.
70genesenriched (specific) in kidney
In the Human Protein Atlas study,397 of all humangenes showed some level of elevated expression in kidney when compared to other tissues. Only 70 were found to be kidney specific (enriched),expressed at least five times more in kidney as compared to other tissues.
Kidney proteins relate to filtration, adsorption and transport
Kidney enriched genes correspond to proteins related to filtration, adsorption of small molecules, readsorption of electrolytes as well as proteins involved in salt and water transport.

The BSDN (Barttin) protein is tissue enriched in kidney, with at least 5 times higher RNA expression in kidney compared to other tissues. This is illustrated here with IHC staining in seven different tissues using theAnti-BSND antibody (HPA053836). No staining is seen in cerebellum (A), liver (B), stomach (C), testis (D), tonsil (E) or vagina (F) tissues, whereas the Anti-BSDN antibody staining in human kidneyshows strong cytoplasmic and membranous positivity in subsets of tubules (G).
Proteins specifically expressed in glomeruli, renal tubules and collecting ducts
Kidney enriched genes encode proteins localized in glomerulus, distal and proximal tubule and collecting duct, the plasma filtering system of the kidney.
Podocin is one example of a well-known glomeruli associated protein, illustrated below using theAnti-NPHS2 antibody. The proximal tubules are where most of the proteins elevated in kidney are localized, hereexemplified with theAnti-SLC22A2 antibody.

Strong membranous positivityin glomeruli in kidney usingAnti-NPHS2 antibody (HPA049486).

Strong immunoreactivity in proximal tubules in kidney usingAnti-SLC22A2 antibody (AMAb90791).
Transmembrane protein 72 is a protein specifically expressed in the distal tubules,shown by theAnti-TMEM72 antibodybelow. After passing through the tubules, the filtrate continues to the collecting duct, here illustratedby theAnti-AQP2 antibody.

Strong cytoplasmic positivity in cells in distal tubules in kidney usingAnti-TMEM72 antibody (HPA062907).

Membranous positivity in collecting ducts in kidney usingAnti-AQP2 antibody (HPA046834).
PLA2R1 in membranous nephropathy
PLA2R1 (M-type phospholipase A2 receptor) is the major antigen in idiopathic membranous nephropathy, a slowly progressive disease of the kidney.
 IF staining of paraffin embedded kidney biopsy specimen from patient with membranous nephrop¬athy using the monoclonalAnti-PLA2R1 antibody (AMAb90775).
IF staining of paraffin embedded kidney biopsy specimen from patient with membranous nephrop¬athy using the monoclonalAnti-PLA2R1 antibody (AMAb90775).
 IHC staining of human kidney with membranous nephropathy shows strong membranous immunoreactivity in renal glomeruli usingAnti-PLA2R1 antibody (AMAb90772).
IHC staining of human kidney with membranous nephropathy shows strong membranous immunoreactivity in renal glomeruli usingAnti-PLA2R1 antibody (AMAb90772).
 IHC staining of normal human kidney shows only low background positivity usingAnti-PLA2R1 antibody (AMAb90772).
IHC staining of normal human kidney shows only low background positivity usingAnti-PLA2R1 antibody (AMAb90772).
References
Debiec H, Ronco P.PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephrop¬athy.N Engl J Med2011 Feb 17;364(7):689-90.
Debiec H et al.Autoantibodies Specific for the Phospholipase A(2) Receptor in Recurrent and De Novo Membranous Nephropathy.Am J Transplant2011 Oct;11(10):2144-52.
IHC staining patterns seen in kidney
 Nuclear positivity in distal tubules in kidney usingAnti-POU3F3 antibody (HPA067151).
Nuclear positivity in distal tubules in kidney usingAnti-POU3F3 antibody (HPA067151).
 Cytoplasmic membranous positivity in proximal tubules in kidney usingAnti-SLC13A3 antibody (HPA014736).
Cytoplasmic membranous positivity in proximal tubules in kidney usingAnti-SLC13A3 antibody (HPA014736).
 Membranous positivity in collecting ducts in kidney usingAnti-FXYD4 antibody (HPA058421).
Membranous positivity in collecting ducts in kidney usingAnti-FXYD4 antibody (HPA058421).
 Staining of proximal and distal tubules in kidney usingAnti-CDH16 antibody (HPA036260).
Staining of proximal and distal tubules in kidney usingAnti-CDH16 antibody (HPA036260).
 Luminal membranous staining in proximal tubules in kidney usingAnti-SLC22A13 antibody (HPA035603).
Luminal membranous staining in proximal tubules in kidney usingAnti-SLC22A13 antibody (HPA035603).
 Luminal membranous staining in distal tubules in kidney usingAnti-SLC12A3 antibody (HPA028748).
Luminal membranous staining in distal tubules in kidney usingAnti-SLC12A3 antibody (HPA028748).
 Basolateral membranous staining of proximal tubules in kidney usingAnti-SLC22A8 antibody (HPA044174).
Basolateral membranous staining of proximal tubules in kidney usingAnti-SLC22A8 antibody (HPA044174).
 Staining of a subset of distal tubules in kidneyAnti-ATP6V1G3 antibody (HPA028701).
Staining of a subset of distal tubules in kidneyAnti-ATP6V1G3 antibody (HPA028701).
 Staining of a subset of distal tubules in kidney usingAnti-PVALB antibody (HPA048536).
Staining of a subset of distal tubules in kidney usingAnti-PVALB antibody (HPA048536).
Free Poster of the Human Cell
Interested in the human cell? Order a free copy of this poster to learn about the most detailed mapping of the human cell ever done.
Explore the Human Cell
TheHuman Protein Atlashascreateda map of the human cell, set to be released on December 4th. In the map, proteins have been localized with high precision to cellular organelles, structures and sub-structures,with high-resolution images freely available for you to explore. The polyclonal antibodies used for this research areavailable from Atlas Antibodies as Triple A Polyclonals.
This poster summarizes the researchand lets you explore the organelle proteomes, multi-localizing proteins, cell cycle dependent proteins and cell line transcriptomes.
300 New IHC Validated Antibodies
Abnova is now offering300new immunohistochemistry (IHC)validated antibodies raised against120+different targets from23biological function categories. Please check the following tables for more information of our newly launched products and empower your research to an advanced level with our high quality antibodies:
New Flow Cytometry Poster
Discover the important steps to success in designing and carrying out a flow cytometry experiment.
How to become an IHC Expert in 4 Days
This guide will show you all the nuts and blots for Immunohistochemistry (IHC), including expert review of principle, optimized protocol that really works and more.
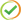 Immunohistochemistry (IHC) principle
Immunohistochemistry (IHC) principle
Having a solid understanding of how IHC works significantly increases your success rate. We have everything you need to know from sample preparation to final counterstain.
 Optimized Protocol
Optimized Protocol
IHC is complex and tricky in many ways. Our step-by-step protocol will guide you to generate reproducible, high quality data.
 Comprehensive Troubleshooting Tips
Comprehensive Troubleshooting Tips
Having our troubleshooting tips that address all kinds of problems can save you a lot of valuable time and headache.
 Frequently asked questions
Frequently asked questions
Learn from your peers’ experiences can bring you novel perspectives and help you avoid common mistakes.
How to become a Western Blot expert in 4 Days
This guide will teach you everything you need to become a Western Blot (WB) expert, including comprehensive principle overview, insightful troubleshooting tips and more.
 Western blot principle
Western blot principle
Having a solid understanding of how Western blot works significantly increases your success rate. We have everything you need to know from sample preparation to data interpretation.
 Optimized Protocol
Optimized Protocol
Western blot is complex and tricky in many ways. Our step-by-step protocol will guide you to generate reproducible, high quality data.
 100+ Troubleshooting Advices
100+ Troubleshooting Advices
Having our comprehensive troubleshooting tips right on your bench side can save you a lot of precious time and headache.
 Frequently asked questions
Frequently asked questions
Learn from your peers’ experiences can bring you novel perspectives and help you avoid common mistakes.
How to become an ELISA Expert in 4 Days
This guide will teach you everything you need to become a ELISA expert, including critical review of principle, all-in-one FAQs and more.
 ELISA principle
ELISA principle
Having a solid understanding of how ELISA works significantly puts you ahead of the learning curve. We have everything you need to know from sample preparation to data analysis.
 Optimized Protocol
Optimized Protocol
ELISA is complex and prone to errors in many ways. Our step-by-step protocol will guide you to generate reproducible, high quality data.
 150+ Troubleshooting Advices
150+ Troubleshooting Advices
Having our troubleshooting guidance that addresses all kinds of problems can save you a lot of precious time and headache.
 Frequently asked questions
Frequently asked questions
Learn from your peers’ experiences can bring you novel perspectives and help you avoid common mistakes.
VEGF Signaling Interactive Pathway
Learn which specific molecules are involved and which phosphorylation sites on VEGF R2 are necessary for promoting several biological effects downstream of VEGF. Molecules in our interactive pathways link directly to products, making it easy to find the reagents you need.
Secondary Antibody Handbook

Everything you need to know
about secondary antibodies

Choose from over 2,500 secondary antibodies conjugated to more than 40 different enzymatic, fluorescent, or biotin labels. Our secondary antibodies are optimized for use in western blot, immunofluorescence, immunohistochemistry, flow cytometry, and more
>Browse Secondary Antibodies
The role of MMP-3 in Human Labor
Commonly known as “breaking of the mother’s waters”, the rupture of fetal membranes is typically one of the final steps that occurs before human labor begins. Matrix Metalloproteinases (MMPs) are heavily involved in restructuring and degrading the extracellular matrix for this process. The specific expression of MMP-9 may denote the onset of labor. Flores-Pliego et al. focused their studies on understanding how placental leukocytes affect MMP levels and activation, which can lead to child birth. BioLegend offers several reagents for MMP study, including the brand new LEGEND MAX™ MMP-3 ELISA Kit.

Adapted from Flores-Pliego, A. et al. 2015. PLoS One. 10:e0145366. Pubmed
It has been noted that a variety of leukocytes arrive in the gestational tissues during the third trimester of pregnancy. These placental leukocytes are known to produce MMP-9 in addition to several other cytokines. Flores-Pliego et al. observed that these isolated cells steadily produced MMP-9 in vitro. They also found that MMP-3, specifically, was upregulated and could induce MMP-9 activation. These elevated MMP levels can then aid in the degradation of fetal membrane components like collagen and proteoglycans, readying the body to give birth.
Updated Flow Buffers Webpage
Beyond antibody reagents, flow cytometry requires the right types of buffers for optimal staining. This convenient, newly stream-lined list separates out flow cytometry applications by their intended target. It also indicates which buffers are best-suited to your task for surface or intracellular staining and the protocols necessary for each.


StressMarq's Guide to Proper Reagent Storage and Handling
Many people don’t take the time to store and handle their reagents properly. This can lead to reagents that do not work as expected, and this can have a huge impact on your experimental results.
Here are 4 golden rules to follow to get the most from your expensive reagents:
1. STORE THEM CORRECTLY
When you receive a new reagent, don’t leave it sitting on your desk for hours or days. Check out the datasheet to see how/where it is supposed to be stored, then do it.
You may think you know how a particular type of reagent is supposed to be stored, but not all products are the same.
Most antibodies and proteins require -20⁰C or -80⁰C, so you may think all antibodies go in the -20⁰C, right? Wrong! Antibodies that have been conjugated need to be stored between 2-8⁰C; that includes conjugated primary and secondary antibodies. Always check the datasheet.
Did your fluorescent antibody or dye come in a clear plastic tube, enabling you to see the beautiful shimmering colour? Whoops!
You should transfer it to an amber (brown) vial or wrap it in tin foil to prevent it from getting photobleached.
Even in the freezer, repeated exposure to light when the door is opened can damage your dye.
2. ALIQUOT, ALIQUOT, ALIQUOT… X 100
Make time when you first receive the product to measure out aliquots.This avoids freeze thaw cycles that can damage your antibodies and proteins.
Also, in the event of contamination, only one aliquot will be ruined, not the whole amount.
Aliquoting also allows for easy sharing of reagents with other labs without giving away the whole vial, which you may never see again! Sharing now also allows you to expect reciprocal sharing in the future!
3. DON’T DOUBLE DIP
Unless you prepared one-time use aliquots, you may risk contaminating your vial by using a pipette tip that has already been dipped into another solution. Sodiscard it, and use a fresh tip every time.
4. OPTIMIZE!
The recommended dilution listed on a datasheet from a manufacturer is usually just that, a recommended dilution.
It isn’t the lowest amount possible that you can use and still see results. It is the concentration at which most users should start to optimize from.
You need to test out every antibody, and every new lot number, to see what is the best dilution for your species, tissue, and application.
It is likely that you can use a much lower concentration than recommended by optimizing the protocol beforehand.
BioAcademia, Inc
BioAcademia, Inc., a producer of high quality polyclonal antibodies is now providing an ultra-fast delivery service from their new European hub, servicing both the European and North American markets. In most scenarios BioAcademia products can now be delivered to your door within 48 hours! BioAcademia boasts tried and tested monoclonal and polyclonal antibodies widely used by academicians world-wide.
ChIP Assay Procedure and Essential Tools
Chromatin Immunoprecipitation assays (ChIP assays) identify links between the genome and the proteome by monitoring transcription regulation through histone modification (epigenetics) or transcription factor:DNA binding interactions. The strength of ChIP assays is their ability to capture a snapshot of specific protein:DNA interactions occurring in a system and to quantitate the interactions using quantitative polymerase chain reaction (qPCR). Chromatin IP experiments require a variety of proteomics and molecular biology methods including crosslinking, cell lysis (protein-DNA extraction), nucleic acid shearing, antibody-based immunoprecipitation, DNA sample clean-up and PCR. Additional techniques such as gel electrophoresis are usually used during optimization experiments to validate specific steps.
A step-by-step guide to successful chromatin immunoprecipitation (ChIP) assays
NEWThis updated overview of the ChIP procedure includes additional detail about primary antibody selection (i.e., ChIP-validated antibodies). The application note also describes and provides examples of chromatin immunoprecipitation (ChIP) as a technique for studying epigenetics, as it allows researchers to capture a snapshot of specific protein–DNA interactions.
Step 1: Crosslinking

Chromatin immunoprecipitation (ChIP) assays begin with covalent stabilization of protein-DNA complexes. Many protein-DNA interactions are transient, and involve multi-protein complexes to orchestrate biological function.In vivocrosslinking covalently stabilizes protein-DNA complexes.
In vivocrosslinking is traditionally achieved with formaldehyde but can be combined with other crosslinkers such as EGS and DSG. Formaldehyde crosslinking is ideal for two molecules which interact directly. However, formaldehyde is a zero-length crosslinker, limiting its functionality. For higher order interactions, longer crosslinkers such as EGS (16.1 Angstroms) or DSG (7.7 Angstroms) can trap larger protein complexes with complex quaternary structure.
Researchers often use a combination of crosslinkers to trap protein-DNA interacting partners. These crosslinkers permeate directly into intact cells to effectively lock protein-DNA complexes together, allowing even transient interaction complexes to be trapped and stabilized for analysis.
Learn more
View products
Protein Interactions Handbook
Our 72-page Protein Interaction Technical Handbook provides protocols and technical and product information to help maximize results for Protein Interaction studies. The handbook provides background, helpful hints and troubleshooting advice for immunoprecipitation and co-immunoprecipitation assays, pull-down assays, Far-Western blotting and crosslinking. The handbook also features an expanded section on method to study protein:nucleic acid interactions, including ChIP, EMSA and RNA EMSA. The handbook is an essential resource for any laboratory studying Protein Interactions.
Contents include: Introduction to Protein Interactions, Co-Immunoprecipitation, Pull-Down Assays, Crosslinking Reagents, Label Transfer, Far-Western Blotting, Protein Interaction Mapping, Yeast Two-hybrid Reporter Assay, Electrophoretic Mobility Shift Assays [EMSA], Chromatin Immunoprecipitation Assays (ChIP), Protein:Nucleic Acid Conjugates, and more.
Step 2: Cell Lysis

The lysis stage extracts the crosslinked protein-DNA complexes from cells or tissue and brings them into solution. At this stage, cellular components are liberated by dissolving the cell membrane with detergent based solutions. Because protein-DNA interactions occur primarily in the nuclear compartment, removing cytosolic protein can help reduce background and increase sensitivity. The presence of detergents or salts will not affect the protein-DNA complex, as the covalent crosslinking achieved in step one will keep the complex stable throughout the ChIP procedure.
Mechanical lysis of cells is not recommended, as it can result in inefficient nuclear lysis. Reagents such as the Thermo Scientific Pierce Chromatin Prep Module, which isolate the nuclear fraction from other cellular components, are used to eliminate background signal and enhance sensitivity.
View products
Step 3: Chromatin Preparation (Shearing/Digestion)

The extraction step yields all nuclear material, which includes unbound nuclear protein, full length chromatin and the crosslinked protein-DNA complexes. In order to analyze protein binding sequences, the extracted genomic DNA must be sheared into smaller, workable pieces. DNA fragmentation is usually achieved either mechanically by sonication or enzymatically by digestion with micrococcal nuclease (MNase).
Ideal chromatin fragments can range from 200 to >1000 bp, however DNA shearing is one of the most difficult steps to control. Sonication provides truly randomized fragments, but limitations include the requirement of dedicated machinery which may need tuning, difficulty in maintaining temperature during sonication and extended hands-on time and extensive optimization steps. Enzymatic digestion with micrococcal nuclease is highly reproducible and more amendable to processing multiple samples, but can lead to variability due to changes in enzyme activity and the enzyme has higher affinity for inter-nucleosome regions.
Learn more
View products
Step 4: Immunoprecipitation

To isolate a specific modified histone, transcription factor or co-factor of interest, ChIP validated antibodies are used to immunoprecipitate and isolate the target from other nuclear components. This step selectively enriches for the protein-DNA complex of interest and eliminates all other unrelated cellular material.
Selection of the appropriate antibody is a critical parameter to successful ChIP assays. For mammalian samples, numerous ChIP-grade antibodies are available that are validated for this procedure. For target proteins in which qualified antibodies are unavailable, fusion proteins such as HA, myc or GST can be expressed in the biological sample and then antibodies against the affinity tags can be used to immunoprecipitate the target.
The antibody-protein-DNA complex is affinity purified using an antibody binding resin such immobilized protein A, protein G or protein A/G. For biotinylated antibodies, immobilized streptavidin or immobilized Thermo Scientific NeutrAvidin Protein can also be used. For reduced background, it is necessary to block antibody binding beads with a combination of nucleic acid and protein blocking buffers such as salmon sperm DNA and a generic protein source. The volume of beads used in each ChIP sample can also influence background, as the increase in bead volume increases non-specific binding.
View products
Step 5: Crosslinking Reversal and DNA Clean-up

Enrichment of DNA bound to the protein of interest is the goal for chromatin immunoprecipitation. DNA levels can be determined by agarose gel electrophoresis or more commonly by quantitative polymerase chain reaction (qPCR). Before the specific DNA products of a chromatin IP can be amplified and measured, the crosslinks between protein and DNA must to be reversed. This is typically done through extensive heat incubations or through digestion of the protein component with proteinase K.
Proteinase K cleaves at the carboxy-side of aliphatic, aromatic or hydrophobic residues. Because of its broad specificity, proteinase K is often used for removing proteins from DNA or RNA preparations. Additionally, proteinase K digestion eliminates nucleases from the purified DNA, which prevents degradation. To separate DNA from protein fragments, phenol-chloroform is used in conjunction with a standard DNA purification method. Alternatively, spin columns designed to purify nucleic acid material from complex biological samples may be used.
View products
Step 6: DNA Quantitation

A hallmark of ChIP is the ability to quantitate the purified DNA products with quantitative PCR. Despite being amplification techniques, qPCR procedures are sufficiently accurate to enable measurement of target protein-DNA levels in different experimental conditions. There is a direct correlation between the amounts of immunoprecipitated complex and bound DNA.
View products
For Research Use Only. Not for use in diagnostic procedures
Western Blot Loading Control Handbook
Your Handbook to
generate acccurate
Western blot expression data

Our new Loading Control Handbook will provide technical guidance on loading control proteins and Western blot normalization. Become an expert and improve the quality of your Western blot data.

Read more about:
• Optimizing your loading
control to generate
accurate expression data.
probe loading control
expression. • Top loading controls
based on target protein
localization.
Make Cell Biology Research a piece of cake
How does BioLegend make it work for you?
- Outstanding value
- QC testing of every lot with the indicated application
- Example data on each technical data sheet
- Unique research tools, such as our Direct-Blot™ HRP conjugated primary antibodies
- 100% satisfaction guarantee
- Find up-to-date productcitationsand end-userreviews.
Request the free Cell Biology brochure.

Creative BioMart Seminar-for Matrix Metallopeptidases Offering
Creative BioMart recently launched a seminar to discuss the development of Matrix Metallopeptidases and the market of it worldwide in the aim of having a better understanding about the market and providing more excellent products for customers after making some changes accordingly. Scientists staff related in Creative BioMart and core marketing staff as well as some customer representatives attended the lecture.
World proceeds and technologies change. So does the biotech field and the market for Matrix Metallopeptidases. The matrix metalloproteinases (MMPs) are one of the major families of proteinases that play key roles in the responses of cells to the microenvironment. MMPs are a family of calcium-dependent endopeptidases that form a subgroup of the metzincins. These proteins are characterized by a conserved motif containing three histidine residues, which bind a zinc ion at the catalytic site. There are about 24 human MMPs, and homologues can be identified in other species including mice, birds, zebrafish, fruit fly, nematodes, plants and even algae.
Linna Green, the chief marketing staff in Creative BioMart said in the seminar that “We found out that the customers’ needs for MMP is increasing, so we decided to enhance our offerings with our current quality assured. We must work hard for early results. Guaranteed by our advanced techniques and experienced staff, we do believe the day is no faraway to come.”
Through this seminar, Creative BioMart got further advance in Matrix Metallopeptidases Offering though it has already stood out both in reputation and product quality.
To know more information about Matrix Metallopeptidases in Creative BioMart, please visit http://www.creativebiomart.net/gene-family-6-mmps.htm
About Creative BioMart
Creative BioMart provides quality recombinant proteins, lysate, interferon, chromatography, TNF, and native proteins to the research community of biology, clinical research, molecular diagnostics and biopharmaceutical drug development. We are offering more than 1,000 recombinant proteins, peptides and antibodies. All the products are rigorously tested to meet the most demanding research needs. At the same time, lowest prices in the industry are always guaranteed.
The power of multiplexing - White paper from Invitrogen
The purpose of this paper is to address questions relevant to researchers who are familiar with enzymelinked immunosorbent assay (ELISA) technology but are considering a multiplex solution such as Invitrogen multiplex assays on the Luminex™ platform to measure multiple proteins simultaneously in one well.
GBSI Antibody Validation Workshop Gathers Key Stakeholder Groups
TheGlobal Biological Standards Institute(GBSI) targets the quality of research antibodies at a workshop at Asilomar next month in its ongoing efforts to improve reproducibility in preclinical research.Antibody Validation: Standards, Policies, and Practicesbrings together 100 leaders representing academia, antibody producers, pharma, funders, journals and policy makers to share perspectives, build consensus and recommend actionable solutions for improving accuracy in research antibody usage and validation. It is the first convening of all such stakeholder groups with the express purpose of developing antibody standards.
WHEN: September 25-27, 2016
Press Briefing Webcast,
September 28, 9:00 a.m. PT; 12:00 p.m. ET
WHERE:Asilomar Conference Grounds
Pacific Grove, California
Research antibodies are indispensable biological reagents in basic and translational laboratories and clinical settings around the world. They are increasingly used in a wide variety of biomedical applications, with global sales of $1.6 billion in 2011 estimated to increase to $2.6 billion by 2019. However, antibody quality varies drastically, and as such they are a major contributor to the life science research reproducibility crisis and currently there are no standardized and widely accepted guidelines for antibody validation.
PROGRAM AND PRESENTERS:
To build consensus for research antibody validation standards, in advance of the workshop, GBSI engaged the scientific community in asurveyas well as an online dialogue—GBSI Antibody Validation Online Discussion Group. The discourse, covering four themes:Creating Antibody Standards, Responsibilities of the Producers, Drivers for Adoption,andThe Future of Antibodies,continues to progress online and will inform the outcomes of the workshop. In addition, preceding conferences addressing antibody validation in Europe and Asia will further build on the topics. Several attendees of those meetings will join the full representation of stakeholder groups meeting at Asilomar.
The workshop, hosted by GBSI and planned in conjunction withThe Antibody Society, offers an ambitiousagendathat includes 15+ forums and breakout sessions addressing:
- The Science Behind Antibody Validation Standards
- Finding Common Ground: Building Consensus
- Producers and Service Providers in a Pivotal Role: QC/QA and Certification
- Integration of Recombinant Antibodies
- Incorporating Antibody Databases
- Introducing Drivers for Adoption from Funders and Journals
- Testing Rigor, Reproducibility, and Antibodies: The View from NIH
- Roadmap for Moving Forward: Action Steps and Assignments
More than 40 leaders and innovators will facilitate the sessions and guide the discourse, including:
- James Anderson, PhD,NIH
- C. Glenn Begley, MBBS, PhD,Akriveia Therapeutics
- Andrew Bradbury, MBBS, PhD, Los Alamos National Laboratory
- Veronique Kiermer, PhD,PLOS Journals
- Joshua LaBaer, MD, PhD, Biodesign Institute at Arizona State University
- Andreas Plückthun, PhD, University of Zurich
- David Rimm, MD, PhD,Yale University
- Mathias Uhlén, PhD,KTH Royal Institute of Technology (Sweden)
PRESS BRIEFING WEBCAST:
A press briefing on Wednesday, Sept. 28 at 9:00 a.m. PT (12:00 p.m. ET) will be webcast.Leonard P. Freedman, PhD, President of GBSI and workshop chair, and other stakeholder experts will provide impressions of the workshop, highlights of the discourse and proposed next steps. Leadership is available for interviews scheduled pre and post workshop, and at limited times throughout the event.
SPONSOR SUPPORT:
GBSI is grateful for workshop funding and other support by sponsors,National Institutes of Health* andThermo Fisher Scientific, (platinum level);The Antibody Society,Abcam,Amgen,Bio-Techne,Gilead, (gold level);BioLegend,Bristol-Myers Squibb,Cell Signaling Technology,Genentech,Rapid Novor, (silver level); andAbbVie,AdipoGen Life Sciences,Association of Biomolecular Resource Facilities,Grifols,OriGeneandRockland Immunochemicals(bronze level).
About Global Biological Standards Institute (GBSI)
GBSI, a non-profit organization, is dedicated to enhancing the quality of biomedical research by advocating best practices and standards to accelerate the translation of research breakthroughs into life-saving therapies. For more information and a list of workshopsteering committeemembers visitGBSI.org;Twitter @GBSIorg.
NK Digital poster
Download this free digital poster
Natural killer (NK) cells are lymphocytes of the innate immune system and show the capacity to kill cancer cells.
Their function is regulated by the balance of inhibitory and activating signals transmitted by several NK cell receptors. Genotyping, phenotyping, and haplotyping of NK cell receptors are important in different allogeneic hematopoietic stem cell transplantation (HSCT) settings. The following poster describes four robust 8-color flow cytometry panels to phenotype NK cell receptors including inhibitory and activating KIRs, KLRs, NCRs and NK cell maturation markers.
The poster highlights the analysis using recombinantly generated REAfinityTMantibodies, which are designed to show high lot to lot consistency and no background binding to FcγRs.
5 Free Image Analysis Software Tools for Microscopy
Check them out here
No Compensation Panels
Although compensation can be relatively straightforward with the appropriate controls, it can still be daunting to know just how much compensation to apply. With this in mind, examples of panels have been created with common surface markers for human peripheral blood that will allow you to identify many populations, such as T cells, B cells, NK and myeloid populations with little or no compensation required. Even though compensation is not required, we still recommend using single stained controls to reveal the biology of each marker for the specific sample.
- General identification panels
These panels allow you to identify T cells, B cells, NK cells and myeloid cells in human peripheral blood. - T cell panels
These panels allow identification of activated, memory and naïve subsets with helper and cytotoxic T cells. - B cell panels
The panels shown here allow identification of naïve, memory, transitional and plasmablast B cells. - NK and myeloid panels
These panels allow identification of NK and NKT cells and various myeloid populations with peripheral blood.
Compensation
When performing multicolor flow cytometry there is a risk of spectral overlap between fluorophores. Although a fluorophore such as FITC has maximal emission at 525nm, it actually emits some photons with higher wavelengths which will pass through other filters and end up being detected by other than the supposed PMTs (photomultiplier tubes; detectors), this is known as spectral overlap. So if FITC and PE (that normally use a filter of 585/40 in front of its PMT) are combined in a panel, the relative contribution of FITC and PE fluorophores in both the FITC and the PE detectors must be determined, this procedure is called compensation (Figure 1).
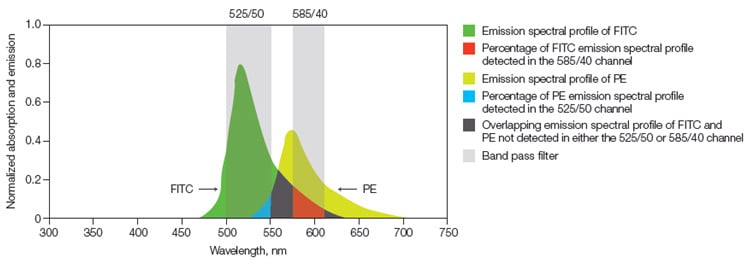
Figure 1.Fluorescence compensation.Emission spectra of two fluorophores commonly used in flow cytometry, FITC and PE are shown with graphic representation of two commonly used filters to detect the fluorophores. The portion shown in red is the proportion of FITC that must be subtracted from PE using compensation.
Compensation is a mathematical method developed to account for spectral overlap and to measure the photons deriving from one fluorophore into multiple detectors, so called false-positive signals. The level of false-positive signals must be determined for each fluorophore individually in all the detectors used in your experiment. This is done by single stained controls that are required for all fluorophores used and will reveal the level of spectral overlap for each detector. When performing compensation the fluorophore in the control needs to be identical to the fluorophore used in the sample, and its brightness should be at least of a similar level. For more information on controls, go to our dedicatedcontrols in flow cytometrywebpage. Figures 2 and 3 showhow compensation can be applied to samples to reveal correct levels of staining.
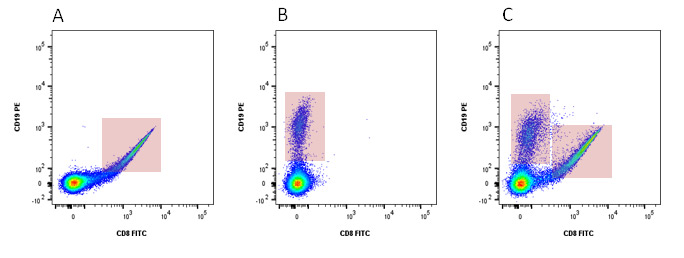
Figure 2. Analyzed without compensation.Peripheral blood was singly stained with CD8 FITC (A) or CD19 PE (B) and both CD8 FITC and CD19 PE (C). In panel A, when compensation was not applied, there is fluorescence spillover into the PE channel from the FITC (marked by the red box). In panel B there is a slight amount of spillover from PE into the FITC channel. Panel C shows how this affects dual staining. Double positive cells are detected even though in this case the markers are mutually exclusive.
Stained cells analyzed with compensation
Figure 3. Analyzed with compensation.When compensation is applied to both FITC and PE channels we can clearly identify the CD19 and CD8 positive populations with no double positive cells (C).
Cell Press Partnership and 2015's Popular Papers by Protein
NEW from Cell Press: At the bottom of each abstract on Cell.com, you will now see a table with extended information about the antibodies used in each article, including direct links to the antibody datasheets. This applies to most articles published since 2011. All articles should be completed within the next few weeks. Please note that this information is currently only available on Cell.com, and not within Elsevier's Science Direct. So, to see this information, please view the article on Cell.com
Because of the specificity of antibodies, this analysis has enabled us to identify which papers feature which proteins. Thus, thisspreadsheetcontains a list of all of the proteins featured in theCell Press (CP) papers analysed so far, and which articles they appear in. The data is ordered by protein, and then within each protein section we have also ordered the list so that the highest impact (most popular) articles are at the top. There are two tabs in the spreadsheet: one for 2015 articles, and one for pre-2015 articles. The impact of each article is based upon the activity around the article throughout 2015.
Researchers tell us that journal articles are their primary source for choosing antibodies, so we hope that this partnership with CPwill make it easier to choose, by highlighting the antibodies which have been through the rigours of the CP peer review system in this stand-alone section, as well as providing extra information about that antibody, and the next best alternative when that antibody is not available.
Your suggestions and comments are welcome and would be appreciated at Contact Us.
Current Comparison reports available
All our available reports can be found here